Answers
Answer:
radiant energy, solar energy
During nuclear fusion, radiant energy and solar energy from the core of the Sun is released. Hope it helps!
Related Questions
Please help ASAP. Thanks so much!!!

Answers
The Keq for the reaction will be 0.89.
The concentration of CO2 Will be 1.67M.
How to calculate the valueThe equilibrium constant (Keq) for the reaction formulated as Keq = [H2O]^2 / ([H2]^2 [O2]), can be evaluated using the given concentrations of hydrogen and oxygen.
After applying both their respective quantities, we arrive at a calculated value of 0.89 for Keq. Moving on, to calculate the concentration of CO2 in the equation H2O (1) + CO2 (g) → H2CO3 (aq), where Keq = [H2CO3] / ([H2O] [CO₂]), having been supplied with a value of Keq equal to 0.15 and H2CO3 being at 0.25M, the rearranged expression reveals that [CO2] equals 1.67 M following basic algebraic substitution.
Learn more about hydrogen on
https://brainly.com/question/24433860
#SPJ1
electronegativity of SO4 and H2SO4
Answers
The electronegativity of SO\(_4\) is more than electronegativity of H\(_2\)SO\(_4\). The atomic number and the separation of the valence electrons from the charged nucleus have an impact on an atom's electronegativity.
What is electronegativity?When an atom of a certain chemical element forms a chemical connection, it has a propensity to draw shared electrons (called electron density), which is represented by the symbol.
The atomic number and the separation of the valence electrons from the charged nucleus have an impact on an atom's electronegativity. The electronegativity of SO\(_4\) is more than electronegativity of H\(_2\)SO\(_4\).
Therefore, the electronegativity of SO\(_4\) is more than electronegativity of H\(_2\)SO\(_4\).
To know more about electronegativity, here:
https://brainly.com/question/13987202
#SPJ9
PLEASE HELP ME ASAP SCREENSHOT DOWN BELOW

Answers
Answer:
Elevation.
Explanation:
Although latitude is a very important factor in temperature, Las Vegas and Mt. Charleston are pretty close to each other, which makes me think that elevation would be the only thing that could explain the extreme difference in temperature.
The length is 3 meters, the width is 9 meters, and the height is 2 meters. What is the
volume?
5.
The length is 10 meters, the width is 4 meters, and the height is 7 meters. What is the
volume?
Answers
Answer:
3 x 9 x 2 = 54 meters
10 x 4 x 7 = 280 meters
Explanation:
Multiply the length, the width, and the height.
You can multiply them in any order to get the same different result. The formula for finding the volume of a rectangular prism is the following: Volume = Length * Height * Width, or V = L * H * W.
Answer:35 cubes with a length of 7 meters, height of 1 meter, and width of 5 meters.
What is the length of the figure?
✔ 7 m
What is the width?
✔ 5 m
What is the height?
✔ 1 m
What is the number of cubes?
✔ 35 cubes
What is the volume?
✔ 35 cubic meters
Explanation:
Explain in complete sentences how heat is transferred in fluids?
Answers
The process of heat transfer in fluids is known as convection and it involves the actual movement of the molecules of the fluids from hotter to cooler regions as a result of decrease in the density of the heated molecules.
What is the name given to the process of heat transfer in fluids?
The name given to the process of heat transfer in fluids is convection.
Convective heat transfer, frequently referred to as convection, is the movement of fluids that transfers heat from one location to another.
In convection, heat energy is carried by the moving fluid. The fluid moves from one area with a high temperature to another with a low temperature. In liquids and gases, it is typically the predominant type of heat transmission.
This particular technique of heat transport combines the conduction (heat diffusion) and advection processes (heat transfer by bulk fluid flow).
Learn more about convection at: https://brainly.com/question/9382711
#SPJ1
What is scalar quantity ?
Answers
Answer:
they physical quantities that are uneffected by changes to a vector space basis
How many valence electrons does group 18 have
Answers
Answer:
group 18 has 8 valence electrons, that's why they are stable.
8!
Explanation:
You're looking at the very last column, right? Noble gases are known for their stability because their valence shells are full! Lucky for you, all you have to do to know the number of valence electrons is look at the column number and take ignore the 1! 16 becomes 6, 18 becomes 8.
Which organelles is found in plant and animal cells and is involved in making cellular energy?
Answers
Answer:
The plant and animal cells are eukaryotic and contain well developed cellular organelles.
The cell membrane, cytoplasm, chromosomes, and mitochondria are the structures that are present in both the plant and the animal cells.
The cell wall and chloroplast are present only in the plant cell.
Mitochondria
Because it is right next to the nucleau
Solution Notes
8. Calculate the molarity of 500 ml of 0.0300 moles of NaOH.
Answers
Answer:
\(\huge\boxed{\sf M = 0.06\ M}\)
Explanation:
Given data:No. of moles = n = 0.03 mol
Volume = v = 500 ml = 0.5 L
Required:Molarity = M = ?
Formula:M = n / v
Solution:Put the given data in the above formula.
M = 0.03 / 0.5
M = 0.06 M\(\rule[225]{225}{2}\)
When cyclohexene is mixed in a test tube with a sulfonitric mixture (h2SO4/HNO3) a pale yellow solution is formed, which suddenly explodes, becoming dark brown. What products are formed and why does this happen?
Answers
When cyclohexene is mixed with a sulfonitric mixture (H2SO4/HNO3), it reacts to form nitrocyclohexane and sulfur dioxide.
This reaction proceeds in two steps. Firstly, cyclohexene undergoes electrophilic addition with the nitronium ion (NO2+), which is generated from the reaction between HNO3 and H2SO4. This results in the formation of nitrocyclohexane, giving the initial pale yellow color to the solution.
In the second step, nitrocyclohexane reacts with the excess sulfuric acid present in the mixture. This step is highly exothermic, releasing a significant amount of energy. The sudden release of energy causes an explosion. The exact mechanism of the explosive reaction is complex, involving the generation of reactive intermediates. It is believed that the reaction proceeds via a radical mechanism, where nitrocyclohexane decomposes into highly reactive nitrogen and carbon-centered radicals. These radicals further react with sulfur dioxide, which is produced in the reaction, to form stable compounds. As a result, the solution turns dark brown after the explosion.
for more such question on cyclohexene
https://brainly.com/question/28559170
#SPJ8
The helium tank has a pressure of 650 torr at 25 degree celsius what will be the pressure if the temperature is tripled?
pa help po
Answers
The helium tank has a pressure of 650 torr at 25 degree Celsius and when the temperature is tripled, the pressure will be approximately 1945.71 torr
To find the new pressure when the temperature is tripled, we can use the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature when the volume and the number of particles remain constant. The ideal gas law is given by the equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to convert the initial temperature of 25 degrees Celsius to Kelvin. Adding 273.15 to the Celsius temperature gives us 298.15 K.
Let's assume that the volume, number of moles, and the gas constant remain constant.
If the temperature is tripled, the new temperature would be 3 times the initial temperature, which is 3 * 298.15 K = 894.45 K.
Now, we can set up a proportion to find the new pressure:
P1 / T1 = P2 / T2
Solving for P2 (the new pressure), we get:
P2 = (P1 * T2) / T1
Plugging in the values, we have:
P2 = (650 torr * 894.45 K) / 298.15 K
Calculating this expression, we find:
P2 ≈ 1945.71 torr
Therefore, when the temperature is tripled, the pressure will be approximately 1945.71 torr.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ11
What the expected outcome is, if the MDS is successfully implemented
Answers
If the MDS (Minimum Data Set) is successfully implemented, several positive outcomes can be expected. The MDS is a standardized assessment tool used in healthcare settings to evaluate the physical, mental, and psychosocial well-being of patients.
Its successful implementation can lead to improved patient care, more efficient resource allocation, and enhanced data analysis.With the MDS in place, healthcare providers can gather consistent and comprehensive data about patients, enabling better understanding of their needs and tailoring of individualized care plans.
This can result in improved treatment outcomes and patient satisfaction. Additionally, the MDS facilitates effective communication and information sharing among healthcare professionals, leading to coordinated care and reduced errors.From a broader perspective, successful implementation of the MDS allows for accurate and reliable data collection, enabling robust research and evidence-based decision-making.
This can contribute to advancements in healthcare practices, policy development, and quality improvement initiatives. Ultimately, the successful implementation of the MDS can enhance patient outcomes, improve healthcare delivery, and drive positive changes in the healthcare system as a whole.
For more such questions on outcomes
https://brainly.com/question/30417322
#SPJ11
draw the radial probability functions r2r2(r) of the following atomic orbitals. be sure to indicate which curve corresponds to which orbital and label your axes.
Answers
The graphs for the radial probability functions r2r2(r) of the atomic orbitals can be made using the information provided below.
The radial probability function (rR2(r)) represents the probability of finding an electron in an orbital at a certain distance from the nucleus. The radial probability function can be used to visualize the size and shape of atomic orbitals. The values of the radial probability function are proportional to the square of the radial wave function.
For s-orbitals (1s, 2s, 3s, 4s), the radial probability functions are spherically symmetrical and centered on the nucleus. They decrease rapidly with increasing distance from the nucleus, reaching zero at a certain distance. The 1s orbital is the smallest and has the highest probability of finding an electron close to the nucleus. The 2s orbital is larger than the 1s orbital and has a lower probability of finding an electron close to the nucleus. Similarly, the 3s and 4s orbitals are larger and have lower probabilities of finding an electron close to the nucleus.
For p-orbitals (3p), the radial probability functions are not spherically symmetrical and have a nodal plane where the probability of finding an electron is zero. They have a maximum probability at a certain distance from the nucleus and decrease rapidly with increasing distance from the nucleus.
For d-orbitals (3d), the radial probability functions have more complex shapes with several nodal planes where the probability of finding an electron is zero. They have maxima and minima at different distances from the nucleus.
For f-orbitals (4f), the radial probability functions have even more complex shapes with multiple nodal planes and regions of maximum and minimum probability. The 4f orbitals are larger and have a lower probability of finding an electron close to the nucleus compared to the 3d orbitals.
The inclusion of relativistic effects in the calculations of the radial probability functions can result in differences in the shapes of the functions for s and f orbitals. However, the inclusion of relativistic effects does not significantly affect the shapes of the p and d orbitals.
Learn more about atomic orbitals here:
https://brainly.com/question/28240666
#SPJ4
The complete question is:
Draw the radial probability functions rR2(r) of the following atomic orbitals Be sure to indicate which curve corresponds to which orbital and label your axes 1s, 2$, & 3s on the same graph b_ 3s, 3p, & 3d on the same graph 4s without relativistic effects and 4s with relativistic effects, on the same graph d. 4f without relativistic effects and 4f with relativistic effects, on the same graph.
Help please !!!!!!!!!!

Answers
what is the wavelength of a substance that has a frequency of 8.85 x 10^12 hz
Answers
The wavelength of a substance that has a frequency of 8.85 x 10^12Hz is 3.39*10^5 m.
What does a wave's frequency mean?Frequency is a unit of measurement for how frequently a recurring event, like a wave, takes place over a specified period. A cycle is one repetition of the repeating pattern. Frequency only exists in moving waves that change their places over time. One method to describe how quickly a wave move is by its frequency.
What is wavelength?
The distance between two consecutive wave crests or troughs is known as the wavelength. The direction of the wave is used to measure it.
Calculation:
Given:
Frequency (f) = 8.85*10^12 Hz
We know that,
Speed of light (c) = 3*10^8 m/s
Wavelength (λ) = c/f
Wavelength (λ) = 3*10^8/8.85*10^12
Wavelength (λ) = 3.39*10^5 meter
To know more about frequency click:
https://brainly.com/question/10728818
#SPJ1
If you added an excess of sodium phosphate dodecahydrate to 4.512 g barium chloride dihydrate, how many grams of barium phosphate could you synthesize
Answers
Answer:
3.35g of Ba3(PO4)2
Explanation:
The balanced chemical equation is given as;
3 BaCl2 + 2 Na3PO4 → 6 NaCl + Ba3(PO4)2
Fro the reaction;
3 mol of BaCl2 (624g) reacts wth 2 mol of Na3PO4 (327.88g) to form 1 mol of Ba3(PO4)2 (601.93g)
Since Na3PO4 is in excess, the limiting reactant is; BaCl2. It would determine how much of Ba3(PO4)2 is formed.
624g of BaCl2 = 601.93g of Ba3(PO4)2
4.512g of BaCl2= xg of Ba3(PO4)2
x = 4.512g * 601.93g / 624g
x = 3.35g of Ba3(PO4)2
The mass of barium phosphate synthesized is 3.612 g.
The equation of the reaction is;
3 BaCl2.2H2O + 2 Na3PO4.12 H2O → 6 NaCl + Ba3(PO4)2 + 14H2O
Molar mass of barium chloride dihydrate = 244.26 g/mol
The number of moles of barium chloride dihydrate = 4.512 g/244.26 g/mol
= 0.018 moles
From the balanced reaction equation shown;
If 3 moles of barium chloride dihydrate yields 1 mole of barium phosphate
0.018 moles of barium chloride dihydrate yields 0.018 moles × 1 mole/3 moles
= 0.006 mole of barium phosphate
Mass of barium phosphate = 602 g/mol × 0.006 mole
= 3.612 g
Learn more: https://brainly.com/question/9743981
Label the equivalence point on the graph of pH versus volume of the titration of a strong acid
and strong base shown below.
a. What volume of base was needed to neutralize the acid?
b. What is the pH at the equivalence point?
c. how does the number of moles of hydro i um ions and hydroxide ions compare at the equivalence point?
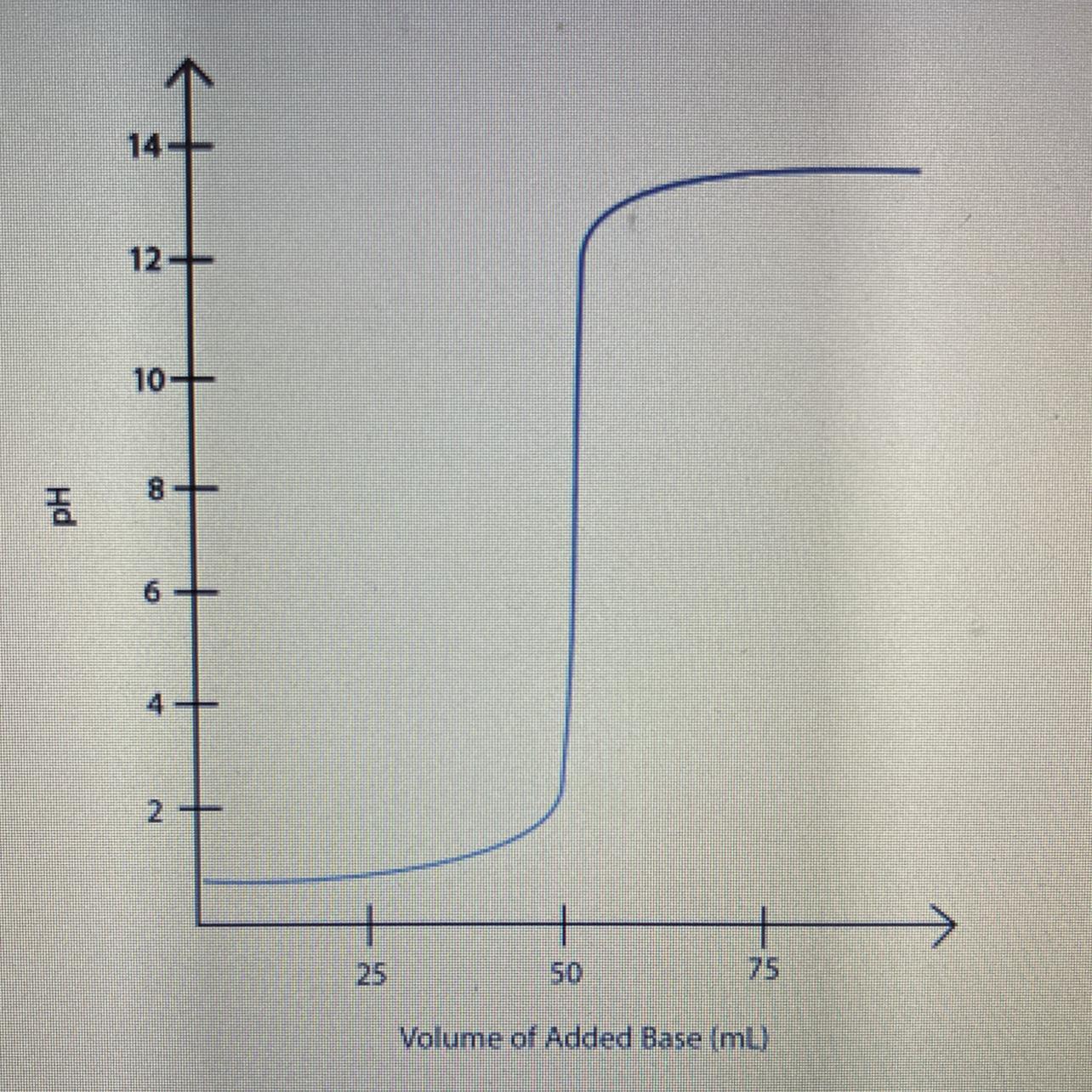
Answers
Based on the titration curve, the titration is of a strong acid strong base and pH at equivalence point is 7.
What is equivalence point?The equivalence point in an acid-base titration is the point at which equal amount of acid and base have reacted.
The equivalence point in a strong acid-strong base titration is at pH 7.
In a weak acid-strong base titration, the pH is greater than 7 at the equivalence point.
In a weak base-strong acid titration, the pH is less than 7 at the equivalence point.
From the titration curve;
volume of base was needed to neutralize the acid is 50 mL pH at the equivalence point is 7the number of moles of hydronium ions and hydroxide ions at the equivalence point are equal.Therefore, the titration curve is of a strong acid strong base.
Learn more about equivalence point at: https://brainly.com/question/24584140
What do you do while drawing a conclusion?
O A. Find a connection between variables
O B. Make a hypothesis
O C. Record observations
D. Make up new data if you need to
Answers
Answer:
A. Find a connection between variables
Explanation:
In the scientific method, the first step is to make an observation. To observe means to carefully monitor phenomena with a view to draw general patterns from specific occurrences.
The second step is to draw up a hypothesis; this is a tentative explanation for the observation.
The next step is to perform an experiment to determine the effect of change in one more variables on another variable. The experiment will confirm or disprove the hypothesis.
The last step is to draw a conclusion. In drawing up a conclusion, a scientist finally establishes the relationship between two variables and finds the connection between them.
A rigid cylinder with a movable piston contains a sample of gas. At 300. K, this sample has a pressure of 240. Kilopascals and a volume of 70.0 milliliter. What is the volume of this sample when the temperature is changed to 150. K and the pressure is changed to 160. Kilopascals?
Answers
Answer:
The volume of this sample when the temperature is changed to 150 K and the pressure is changed to 160 kPa is 52.5 mL.
Explanation:
Boyle's law says that: "The volume occupied by a certain gaseous mass at constant temperature is inversely proportional to pressure" and is expressed mathematically as:
P * V = k
where k is a constant.
Charles's Law consists of the relationship that exists between the volume and the temperature of a certain quantity of ideal gas, which is maintained at a constant pressure, by means of a constant of proportionality that is applied directly. So Charles's law is a law that mathematically says that when the amount of gas and pressure are kept constant, the quotient that exists between the volume and the temperature will always have the same value:
\(\frac{V}{T}=k\)
Gay-Lussac's law states that the pressure of a fixed volume of a gas is directly proportional to its temperature. In other words, if the volume of a certain quantity of ideal gas remains constant, the quotient between pressure and temperature remains constant:
\(\frac{P}{T}=k\)
Combined law equation is the combination of three gas laws called Boyle's, Charlie's and Gay-Lusac's law:
\(\frac{P*V}{T}=k\)
Considering an initial state 1 and a final state 2, it is satisfied:
\(\frac{P1*V1}{T1}=\frac{P2*V2}{T2}\)
In this case:
P1: 240 kPaV1: 70 mLT1: 300 KP2: 160 kPaV2: ?T2: 150 KReplacing:
\(\frac{240 kPa*70 mL}{300 K}=\frac{160 kPa*V2}{150 K}\)
Solving:
\(V2=\frac{150 K}{160 kPa} *\frac{240 kPa*70 mL}{300 K}\)
V2= 52.5 mL
The volume of this sample when the temperature is changed to 150 K and the pressure is changed to 160 kPa is 52.5 mL.
The volume of this sample when the temperature is changed to 150. K and the pressure is changed to 160. Kilopascals is 52.5 mL.
The calculation is as follows:\( \frac{240kPa \times 70 mL}{300K} = \frac{160kPa\timesV2}{150K}\\\\ V2 = \frac{150K}{1600KPa} \times \frac{240kPa \times 70 mL}{300K} \)
So, Volume is 52.5 mL.
Learn more: https://brainly.com/question/26115859?referrer=searchResults
The blue colour of the sky results from the scattering of sunlight by air molecules. Blue light has a frequency of about 7.5*10^14 Hz
Calculate the energy of a mole of photon with this frequency
Answers
Answer:
The energy of a mole of photon is 2.99x10⁵ J.
Explanation:
First, we need to remember that in 1 mol of photons we have 6.02x10²³ photons.
The energy is given by:
\( E = Nh\nu \)
Where:
h: is the Plank's constant = 6.62x10⁻³⁴ J.s
ν: is the frequency = 7.5x10¹⁴ Hz
N: is the number of photons = 6.02x10²³ photons
Hence, the energy of a mole of photon is:
\( E = Nh\nu = 6.02 \cdot 10^{23}*6.62 \cdot 10^{-34} J*s*7.5 \cdot 10^{14} Hz = 2.99 \cdot 10^{5} J \)
I hope it helps you!
PLEASE HELP ME !!!!!!!!!!!!!!
Name: Period; Date:
Problem:
Determine the amount of energy (heat) in Joules and Kcal required to raise the temperature 0f 98.5 grams water from 37.0 0C to 75.0 0C.
Restate the problem (in your own words & all relevant information included).
Model that best represents the problem. Draw and label.
Steps to solve the problem (include formula, known and unknown variables, etc)
Solution and final answer. (show cancellation of units).
Reflection: Must at least have 3 sentences.
Answers
Answer:
Explanation:
Is carbon an organic compound?
A.
Yes – it contains carbon
B.
Yes – all compounds are organic
C.
The question can't be answered with the information given.
D.
No – it is not a compound: it's an element.
Answers
carbon is an elements ,The compounds of carbon are called an organic compounds.
Carbon have tendency to catenation. catenation means forming bond with another carbon atom and form a long chain of carbon. This is the reason for the presence variety of number of organic compounds. The atomic size of carbon is small and have the valency of four, forms covalent bond. Carbon is strong and stable. Generally carbon containing compound are organic compounds. Generally all the Organic compounds contain carbon and hydrogen called as hydrocarbon.
Thus, carbon is an elements ,The compounds of carbon are called an organic compounds.
To learn more about Organic compound here
https://brainly.com/question/4059093
#SPJ1
how many atoms are in 12.0g of carbon
Answers
12 grams of carbon contains 6.022 x 10^23 atoms.
What is the Avogadro's number?
Avogadro's number gives the number of particles in one mole (or moles) of a substance. These particles can be electrons or molecules or atoms. The value of Avogadro's number is approximately 6.022140857 × 10^23 mol−1.
12 grams of carbon contains 6.022 x 10^23 atoms, which corresponds to 1 mole of atoms.
This means that the same number of atoms can be found in other substances of the same weight. Avogadro's number expresses the number of atoms in gram atoms of an element, or the number of molecules in gram moles of a compound.Dividing the atomic mass of an element by the actual mass of that atom gives the value 6.022 xx 10^(23).For example, a carbon molecule contains the same number of atoms as 12 grams of carbon, so 12 grams of carbon equals 6.022 x 10^23 atoms.Therefore, 12 grams of carbon contains 6.022 x 10^23 atoms.
To learn more about Avogadro's numbers, click on the given link:
https://brainly.com/question/11907018
#SPJ1
How many grams of Ag2S2O3 form when 125.0 g AgBr reacts completely according to the reaction below

Answers
The reaction as given is
Na2S2O3 + AgBr ==> NaBr + Na3[Ag(S2O3)2]
Step 1: Balance the equation
2Na2S2O3 + AgBr ==> Na3[Ag(S2O3)2] + NaBr
Step 2: Calculate moles of AgBr present
42.7 g AgBr x 1 mole AgBr/187.8 g = 0.227 moles AgBr
Step 3: Use the mole ratio of the balanced equation to calculate moles Na2S2O3 needed
The mole ratio of Na2S2O3 to AgBr is 2:1
0.227 moles AgBr x 2 moles Na2S2O3 / mole AgBr = 0.454 moles Na2S2O3 needed (answer to part 1)
Step 4: Use the mole ratio and molar mass of NaBr to calculate mass of NaBr produced
0.227 moles AgBr x 1 mol NaBr/mol AgBr x 103 g NaBr/mol NaBr = 23.4 g NaBr produced (answer to part 2)
To know more about silver bromide use link below:
https://brainly.com/question/16958040
#SPJ1
Answer:109.13
Explanation:
Calculate the sublimation pressure of the solid at the melting point of 400.00 K assuming that the enthalpy of sublimation is not a function of temperature.
Answers
This question is incomplete, the complete question is;
Tonksite is a solid at 300.00K. At 300.00 K its enthalpy of sublimation is 66.00 kJ/mol. The sublimation pressure at 300.00 K is 5.00 × 10⁻⁴ atm
Calculate the sublimation pressure of the solid at the melting point of 400.00 K assuming that the enthalpy of sublimation is not a function of temperature.
Answer: the sublimation pressure of the solid at the melting point is 0.3727 atm
Explanation:
Given that;
T1 = 300 K
T2 = 400 K
H_sub = 66 kJ/mol = 66000 J/mol
P1 = 5.00 × 10⁻⁴ atm
p2 = ?
now using the expression
log( p2 / 5.00 × 10⁻⁴ ) = (H_sub / R × 2.303 ) (( T2 - T1) / T1T2)
now we substitute of given values into the expression
log(p2/p1) = (66000 / 8.314 × 2.303 ) (( 400 - 300) / 300 × 400 )
p2 = 0.3727 atm
therefore the sublimation pressure of the solid at the melting point is 0.3727 atm
40 POINTS
Sort the five layers of the atmosphere with the highest level first.
stratosphere
mesosphere
troposphere
ionosphere
thermosphere
Answers
Answer:
troposphere
stratosphere
mesosphere
thermospjere
exosphere
Explanation:
this is from the lowest to highest
I NEED HELP!!OMG OMG OMG

Answers
Winter is purple, spring is blue, summer is orange, fall is green.
b. After 20,000 L of ethylene oxide at 748 kPa and 525 K is cooled to 293 K it is transferred to a 110,000 L tank. what is the new pressure?
Answers
Answer:
75.9 KPa
Explanation:
From the question given above, the following data were obtained:
Initial volume (V1) 20000 L
Initial pressure (P1) = 748 kPa
Initial temperature (T1) = 525 K
New temperature (T2) = 293 K
New volume (V2) = 110000 L
New pressure (P2) =?
The new pressure pressure of the gas can be obtained by using the general gas formula as illustrated below:
P1V1 / T1 = P2V2 / T2
748 × 20000 / 525 = P2 × 110000 / 293
14960000 / 525 = P2 × 110000 / 293
Cross multiply
P2 × 110000 × 525 = 14960000 × 293
P2 × 57750000 = 4383280000
Divide both side by 57750000
P2 = 4383280000 / 57750000
P2 = 75.9 KPa
Thus, the new pressure of the gas is 75.9 KPa
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
The coordination number for Mg2 + ion is usually six. Assuming this assumption holds, determine the anion coordination number in the following compounds: (a) MgS, (b) MgF2, (c) MgO.
Answers
Magnesium Oxide has an anion coordination number of 6, MgS has an anion coordination number of 6, MgF2 has an anion coordination number of 3, and MgS has an anion coordination number of 6.
For Mg2 + ion, six is typically the coordination number. From the viewpoint of Cl, the same is valid. Both ions have a 6 as their coordination number. In a crystal, cations and anions have a molecular ratio of 1:1, which indicates that they share the same coordination number. However, this does not imply that the number is fixed at 6. With the chemical formula MgF2, magnesium fluoride is an inorganic substance. The substance, which has commercial applications in optics and is also utilized in space observatories, is a white crystalline salt that is transparent throughout a broad range of wavelengths known as Magnesium Oxide.
Learn more about Magnesium Oxide here
https://brainly.com/question/1533548
#SPJ4