Write the nuclear equation for the beta decay
of calcium-45.
Answers
Answer:
on https://www.ck12.org/book/peoples-physics-concepts/section/20.1/
Explanation:
it shows for calcium 45 and maybe a possible equation on 3/4th's scroll down?
Related Questions
two. Power Generation Type Nuclear Geothermal Renewable or Non-renewable? paring the Reason For Choice 21. Find the energy released or absorbed when one mole of helium-4 is produced in this reaction. Show your work including any formulas use, substitutions, and record your answer to the correct number of significant digits including the appropriate unit. Also include whether the reaction released or absorbed energy. He + H He + H+ 'n -
Answers
The energy released when one mole of helium-4 is produced in the reaction H + H → He + H⁺ + n is approximately -3.598 × 10⁻¹² Joules per mole.
The reaction of producing one mole of helium-4 (He) from a hydrogen atom (H) and a hydrogen ion (H⁺) with the release of a neutron (n) can be represented as:
H + H → He + H⁺ + n
The energy released or absorbed in this reaction can be calculated using the mass defect method.
The mass of one mole of helium-4 (He) is approximately 4.0026 g/mol, and the mass of one mole of a hydrogen atom (H) and a hydrogen ion (H⁺) is approximately 1.0078 g/mol each. The mass of a neutron (n) is approximately 1.0087 g/mol.
Using these values, we can calculate the energy released or absorbed:
Mass of reactants = 2 × (1.0078 g/mol) = 2.0156 g/mol
Mass of products = 4.0026 g/mol + 1.0078 g/mol + 1.0087 g/mol = 6.0191 g/mol
Mass defect = Mass of reactants - Mass of products = 2.0156 g/mol - 6.0191 g/mol = -4.0035 g/mol
Energy released or absorbed (E) = Mass defect × (c²)
E = -4.0035 g/mol × (2.998 × 10⁸ m/s)²
E ≈ -3.598 × 10⁻¹² J/mol
learn more about mass defect here:
https://brainly.com/question/11624098
#SPJ4
How many electrons did chlorine gain to become an atom with -1 charge ?
Answers
Answer:
17 electrons. On the right, the chloride ion has 18 electrons and has a 1− charge.
Explanation:
one gallon milk is equal to how many milliliters of milk?
Answers
Answer:
3785.411784 mL
Explanation:
Melting point of a substance is what kind of property?
O extensive
O intensive
O chemical
O commercial

Answers
Answer:
A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. Therefore the answer is chemical.
Explanation:
Brainliest?
The homogeneity of the chloride level in a water sample from a lake was tested by analyzing portions drawn from the top and from near the bottom of the lake, with the following results
Top (ppm Cl)
Bottom (ppm Cl)
26.30
26.22
26.43
26.32
26.28
26.20
26.19
26.11
26.49
26.42
Apply the t-test at the 95% confidence level to determine if the chloride level from the top of the lake is different from that at the bottom.
Now use the paired t-test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level.
Why is a different conclusion drawn from using the paired t- test than from just pooling the data and using the normal t- test for differences in means?
Answers
The paired t-test yields a different conclusion than the normal t-test because it accounts for the paired nature of the data, comparing the measurements taken at the top and bottom of the lake separately.
In this scenario, the paired t-test is appropriate because it analyzes the data as pairs, considering the chloride levels measured at the top and bottom of the lake for each sample. By comparing the differences within each pair, the paired t-test determines whether there is a significant difference between the chloride levels at the top and bottom of the lake.
Using the paired t-test, the differences between the paired observations are calculated, and the null hypothesis assumes that the mean difference is zero (no significant difference between the top and bottom chloride levels). The test then determines whether the observed differences are statistically significant at a chosen confidence level, in this case, 95%.
The normal t-test for differences in means, on the other hand, would treat the top and bottom chloride levels as separate and unrelated groups, disregarding their paired nature. By pooling the data and conducting a standard t-test, the analysis assumes that the two sets of measurements are independent, which may not be appropriate in this case. This can lead to a different conclusion compared to the paired t-test.
The different conclusion drawn from using the paired t-test compared to pooling the data and using the normal t-test is due to the consideration of the paired nature of the measurements. The paired t-test takes into account the potential correlation or connection between the measurements taken at the same location (top and bottom of the lake) and assesses the differences within each pair.
Pooling the data and using the normal t-test treats the measurements as independent, disregarding the pairing. This can result in a loss of valuable information and may lead to an inaccurate conclusion. The paired t-test is more appropriate when dealing with dependent or related measurements, ensuring a more accurate assessment of the differences between the top and bottom chloride levels.
Learn more about paired t-test
brainly.com/question/32245864
#SPJ11
The half-life of tritium (h) is 12 years. how long does it take for 16.0 ng of tritium to decay to the point where 2.0 ng remains?
Answers
It takes 24 years for 16.0 ng of tritium to decay to the point where 2.0 ng remains.
Tritium has a half-life of 12 years, which means that in each 12-year period, half of the tritium atoms will decay. To calculate the time it takes for a specific amount of tritium to decay, we can use the concept of half-life.
Step 1:
In the first 12 years, half of the tritium will decay, leaving 8.0 ng remaining (16.0 ng / 2).
Step 2:
In the next 12 years (the second half-life), half of the remaining tritium will decay again, leaving 4.0 ng (8.0 ng / 2).
Step 3:
Continuing this pattern, after another 12 years (the third half-life), we have 2.0 ng remaining (4.0 ng / 2).
Therefore, it takes 24 years for 16.0 ng of tritium to decay to the point where 2.0 ng remains.
Learn more about decay
https://brainly.com/question/32086007
#SPJ11
Why do I always resort to brainly when I know it's kind of wrong-ish........?
The answers are rarely correct and the system is pretty faulty. Is anyone else addicted for no reason? This is for a "Science research project"
Answers
Answer:
Because the thought of self-doubt and lack of confidence is getting to you. Other people most likely have the same issue that you do.
Explanation:
Answer:
i like to use it so i can find out new things, my dad thought me that by teaching you learn more, but when it comes to getting answers i put out a few of the same question and if 2 ppl give the same answer i use that one. the reason why i use brainly is because i have looked everywhere and cant find the answer this way even if its wrong it looks like i tried. i hopes that helps
imagine you are frosting a cake apply pascal's law to using the bag of frosting what would happen when you squeeze the bag
Answers
Why can density be used to find volume of foil
Answers
Answer:
Since the foil has a mass of 830 g, you can use the density to find its volume 830g ⋅ 1 cm3 2.70g = 307.4 cm3 You now know that the volume of the foil is
Explanation:
The density (more precisely, the volumetric mass density; also known as specific mass), of a substance is its mass per unit volume. The symbol most often used for density is ρ (the lower case Greek letter rho), although the Latin letter D can also be used. Mathematically, density is defined as mass divided by volume: ρ=m/V where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight.
is Cu2S covalent or ionic
Answers
Explanation: because it’s in between metal and nonmetal
Cu2S, also known as copper(I) sulfide, is an ionic compound.
Ionic compounds are formed through the transfer of electrons between atoms. In the case of Cu2S, copper (Cu) is a metal, and sulfur (S) is a nonmetal. Metals tend to lose electrons to attain a stable electron configuration, while nonmetals tend to gain electrons.
In the formation of Cu2S, copper loses two electrons to form Cu+ ions, and sulfur gains two electrons to form S2- ions.
The resulting Cu+ and S2- ions are held together by electrostatic forces of attraction due to their opposite charges. This electrostatic attraction between the ions forms the ionic bond. In an ionic compound like Cu2S, the atoms are arranged in a crystal lattice structure.
Therefore, Cu2S is classified as an ionic compound.
Know more about Ionic compounds:
https://brainly.com/question/30420333
#SPJ6
A chemist reacted 15.0 liters of F2 gas with NaCl in the laboratory to form Cl2 and NaF. Use the ideal gas law equation to determine the mass of NaCl that reacted with F2 at 280. K and 1.50 atm.
F2 + 2NaCl → Cl2 + 2NaF
Answers
The mass of NaCl that reacted with F2 gas is approximately 202 grams.
To determine the mass of NaCl that reacted with F2 gas, we can use the ideal gas law equation, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. In this case, we are given the volume of F2 gas (15.0 liters), the temperature (280 K), and the pressure (1.50 atm).
First, we need to convert the volume of F2 gas to moles using the ideal gas law equation. Rearranging the equation to solve for moles, we have n = PV / RT. Substituting the given values, we get
n = \((1.50 atm * 15.0 L) / (0.0821 atm L/mol K * 280 K)\).
Calculating this, we find that the number of moles of \(F_2\) gas is approximately 0.804 moles.
From the balanced chemical equation, we can see that 1 mole of\(F_2\) gas reacts with 2 moles of NaCl. Therefore, the number of moles of NaCl that reacted with F2 gas is half the number of moles of \(F_2\) gas, which is 0.402 moles.
Finally, we can calculate the mass of NaCl using its molar mass. The molar mass of NaCl is 58.44 g/mol. Multiplying the number of moles (0.402 moles) by the molar mass, we find that the mass of NaCl that reacted with \(F_2\) gas is approximately 202 grams.
Learn more about gas law
brainly.com/question/30458409
#SPJ11
PLZ PLZ PLZ PLZ HELPPPP!

Answers
Answer:
Ridges
Explanation:
Ridges separate watersheds
Hope this helped!
Which statement describes a gas condensing into a liquid?
A.
The atoms in the molecules get heavier so they move less.
B.
The molecules break into smaller pieces that take up less space.
C.
The molecules stick together to make bigger molecules.
D.
The molecules get closer together and move more slowly.
Answers
Answer: molecules get closer
Explanation:
Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. When gas molecules transfer their energy to something cooler, they slow down and their attractions cause them to bond to become a liquid
hope this helps
pls mark me brainliest
Answer:
D. The molecules get closer together and move more slowly.
Classify each of these as a heterogeneous or homogeneous mixture

Answers
Answer:
blood- homogeneous
ocean water - heterogeneous
air- homogeneous
blueberry pancakes - homogeneous
milk - homogeneous
steel - homogeneous
A solution of HCl has a concentration of 3.4 x 10-3 M. What would be the [OH-] of this solution?
Answers
Answer:
a
Explanation:
uffvyffvfvf
true or false: in terms of the percentage of alcohol content by volume, one ounce of whiskey has more alcohol than one ounce of wine.
Answers
More wine can be consumed because it has less alcohol than whisky. Wine normally has an alcohol content between 12% and 14%, however, whisky can have anywhere between 40% and 50%. The given statement is true.
Alcohol by volume, also known as ABV, is the proportion of ethanol in a particular volume of liquid. The international unit of measurement for alcohol concentration is the ABV. Unfortified wine has an average alcohol by volume (ABV) of 11.6%, with a range of roughly 5.5% to 16%.
Whisky undergoes fermentation in charred white oak wood to produce its distinct flavor. Whisky ceases aging once it is removed from the casks and bottled. About 40% of good whisky is alcohol.
To know more about alcohol, visit;
https://brainly.com/question/31868718
#SPJ12
1. What two things reflect some of the incoming solar radiation back out into space?
Answers
Answer:
About 29 percent of the solar energy that arrives at the top of the atmosphere is reflected back to space by clouds, atmospheric particles, or bright ground surfaces like sea ice and snow
Jonathan used the following soil triangle to identify a sample of soil as sandy clay. Which description of soil likely allowed Jonathan to make this identification.
A. Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt.
B. Mostly large grains, with a gritty texture, 45% sand, 5% clay, 45% silt.
C. Mostly small grains, with a smooth texture, 30% sand, 5% clay, 65% silt.
D. Mostly small grains, with a sticky texture, 30% sand, 50% clay, 20% silt.
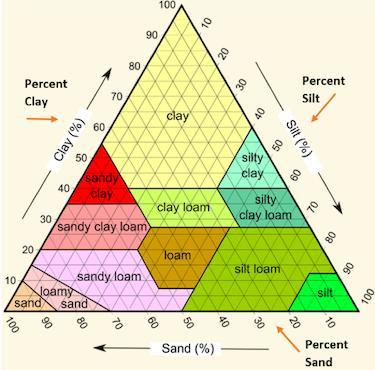
Answers
Answer:
Mostly large grains, with a sticky texture, 55% sand, 40% clay, and 5% silt
Explanation: I took the test, hope it helps.
Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt. The correct option is A.
What is soil triangle?One side of the triangle represents sand, the other side represents clay, and the third side represents silt.
A soil texture triangle is used to classify a soil's texture class. The percentages of sand, silt, and clay are scaled on the sides of the soil texture triangle.
Clay percentages are read across the triangle from left to right. Silt is read from top to bottom, upper right to bottom.
Jonathan used the soil triangle to identify a sample of soil as sandy clay; the soil description that allowed Jonathan to make this identification is 55% sand, 40% clay, and 5% silt, with mostly large grains and a sticky texture.
Thus, the correct option is A.
For more details regarding soil triangle, visit:
https://brainly.com/question/1698808
#SPJ2
What is the force needed to cause a 80kg object to accelerate at 1.6m/s??
Answers
Answer:
The answer is 128 NExplanation:
The force acting on an object given it's mass and acceleration can be found by using the formula
force = mass × acceleration
From the question we have
force = 80 × 1.6
We have the final answer as
128 NHope this helps you
Atoms in aluminium foil paper are the same as the atoms in the aluminium pot.
True or false
Answers
Answer:
False
Explanation:
If each mole contains 6.023 x 10^23 atoms of magnesium. How many magnesium atoms reacted in your reaction? 400 mg of Magnesium were used.
Answers
Answer:
0.1× Avogadro's numbers
10×10^22
Explanation:
convert given mass to moles
then multiply by Avogadro's number
which of the following best describes a functional group? multiple choice question. large molecules comprised of a glycerol and three fatty acid chains large molecules composed of two or more repeating smaller units special combinations of atoms that attach to hydrocarbon chains and rings to form organic nutrients molecules that are composed of hydrogen and carbon in a long chain or ring-like structure
Answers
The answer is (b). Specialized atom combinations known as functional groups bind to hydrocarbon chains and rings to create organic nutrition.
Any of the countless atom combinations that compose chemical compounds and undertake distinctive reactions on their own are categorized as functional groups. It frequently affects the remaining molecules in each molecule's reactivity. The idea of functional groups can be used as a foundation for categorizing numerous substances based on how they behave. Common functional group examples include the hydroxyl found in alcohols and phenols, the carboxyl found in carboxylic acids, and the carbonyl found in aldehydes, ketones, and quinones. It can be simply defined as an atom or group of atoms inside a molecule that share chemical characteristics when they appear in multiple compounds, despite the fact that other components of the molecule are very different.
Learn more about functional groups here
https://brainly.com/question/30666298
#SPJ4
The Complete question is
which of the following best describes a functional group? multiple choice question. large molecules comprised of a glycerol and three fatty acid chains large molecules composed of two or more repeating smaller units special combinations of atoms that attach to hydrocarbon chains and rings to form organic nutrients molecules that are composed of hydrogen and carbon in a long chain or ring-like structure
(a) large molecules comprised of a glycerol and three fatty acid chains
(b) special combinations of atoms that attach to hydrocarbon chains and rings to form organic nutrients
(c) molecules that are composed of hydrogen and carbon in a long chain or ring-like structure
(d) large molecules composed of two or more repeating smaller units
A science class investigated the relationship between the amount of time
students spend doing chores every night and their average grades on
schoolwork. Which statement from the lab report best represents a
conclusion?
A. Based on our results, students who do 1 hour of chores per night
have grades that are on average 0.2 points higher than students
who do no chores.
B. Doing chores at night actually helps students do better at school,
even if it seems like the opposite would be true.
C. We believe that students should spend at least 2 hours doing
chores every night if they want to get better grades at school and
help their families.
O D. If students spend at least 1 hour per night on chores, then they are
guaranteed to get higher grades on their schoolwork.
Answers
Answer:
A
Explanation:
Did the quiz
The statement from the lab report best represents a conclusion, based on our results, students who do 1 hour of chores per night have grades that are on average 0.2 points higher than students who do no chores. The correct option is A.
What is a scientific investigation?A scientific investigation is an investigation that is done by experimenting or solving things by scientific methods. These methods include variables, and controlled calculation and time.
Here, the lab investigation is saying the relationship between the number of times students are spending the time doing chores every night
Thus, the correct option is A. According to our findings, pupils who perform one hour of chores each night earn, on average, 0.2 points more than those who do not.
To learn more about the scientific investigation, refer to the link:
https://brainly.com/question/8386821
#SPJ2
Which word describes how light energy passes through Earth's atmosphere?
Answers
Electromagnetic radiation is reflected or absorbed mainly by several gases in the Earth's atmosphere, among the most important being water vapor, carbon dioxide, and ozone. Some radiation, such as visible light, largely passes (is transmitted) through the atmosphere.
Compare and contrast one mole of a compound and Avogadro's number of particles in a compound. What is the conversion factor between the two?
(Not confident on this. Can someone explain please?)
Answers
The number of particles in a substance is given by the Avogadro's number.
The number of particles in a substance is given by the Avogadro's number. The term "mole" was coined by Avogadro in order to assign a numerical value to the number of particles in a substance.
According to Avogadro, one mole of a substance contains 6.02 × 10^23 particles. These particles may be ions, molecules, atoms etc. This value is otherwise known as the Avogadro's number.
Learn more about Avogadro's number: https://brainly.com/question/1445383
if it takes 35.0 ml of a 0.1 m hcl solution to neutralize 25.0 ml of a naoh solution, what is the concentration of the base?
Answers
With 35.0 mL of a 0.1 M HCl solution neutralizing 25.0 mL of the NaOH solution, we find that the moles of NaOH are equal to the moles of HCl. Using this information, we calculate the molarity of NaOH to be 0.14 M.
To determine the concentration of the NaOH solution, we can use the concept of stoichiometry and the volume and concentration of the HCl solution used in the neutralization reaction. The balanced chemical equation for the reaction between HCl and NaOH is:
HCl + NaOH → NaCl + \(H_{2} O\)
From the equation, we can see that the mole ratio between HCl and NaOH is 1:1. This means that the number of moles of HCl used in the reaction is equal to the number of moles of NaOH present in the solution.
Given that it takes 35.0 mL of a 0.1 M HCl solution to neutralize 25.0 mL of the NaOH solution, we can use the equation:
Moles of HCl = Molarity of HCl × Volume of HCl solution
Moles of NaOH = Moles of HCl
Molarity of NaOH = Moles of NaOH / Volume of NaOH solution
Substituting the given values:
Moles of HCl = 0.1 M × 35.0 mL = 3.5 mmol
Moles of NaOH = 3.5 mmol
Volume of NaOH solution = 25.0 mL = 0.025 L
Molarity of NaOH = (3.5 mmol) / (0.025 L) = 140.0 mmol/L = 0.14 M
Therefore, the concentration of the NaOH solution is 0.14 M.
Learn more about molarity here:
https://brainly.com/question/31545539
#SPJ11
How many chromium atoms are
present in one formula unit of
sodium chromate?
Na2CrO4
A. 2 atoms
B.7 atoms
C. 1 atoms
D. 4 atoms
Answers
Only 1 atom of chromium is present in one formula unit of sodium chromate (option C).
What is a chemical compound?A chemical compound is a substance formed by the union of two or more chemical elements in a fixed ratio, the union being a chemical bond.
A chemical formula, which is a notation indicating the number of atoms of each element present in a compound, shows the type and number of atoms of elements in a compound.
According to this question, a chemical compound named sodium chromate (Na₂CrO₄) is given. This chemical formula shows that only one atom of chromium is present in the compound.
Learn more about chemical formula at: https://brainly.com/question/19474590
#SPJ1
Answer:
D. 4 atoms
Explanation:
You can calculate the mechanical energy of an object by _____.
A. first calculating its thermal energy
B.adding its chemical energy and its electrical energy
C. subtracting its potential energy from its kinetic energy
D. combining its potential energy with its kinetic energy
Answers
Answer:
D - combining it's potential energy with it's kinetic energy
What are two physical properties of lead?
Answers
Answer:
low melting point, high density, and acid resistance
Explanation:
How many grams of Sodium metal are required to completely react with 100 g of Magnesium chloride hexahydrate in a single replacement reaction? (Write a balanced equation first. You don’t need the hydrate part in the balanced equation, you only need it when you do the molar mass of the given).
Answers
Answer:
MgCl2 - 6H2O
203.3 mol
MgCl2 + 2Na = 2NaCl + Mg
22.6g
Explanation:
1. Write the formula of hydrate and find molar mass.
2. Write a balanced single replacement (don't include H2O)
3. Do the given/wanted calculation (include H2O in molar mass of given)