Answers
NH3 i.e. ammonia compounds has a number of electron rich regions that is different from the other compounds.
Hydrides that are electron-rich have more electrons than are required for bonding. The majority of the additional electrons are found on the central atom's single pair of electrons. These kinds of compounds are typically produced by the combination of groups of 15, 16, and 17 elements. Think about NH3, PH3, etc. specific electron. Electron-specific hydrogen molecules have enough valence electrons to form covalent bonds. Those hydrides that possess precisely the proper number of electrons for a covalent connection are known as electron precise hydrides. These kinds of compounds are typically made with group 14 components. The compounds typically have a tetrahedral shape. CH4, SiH4, as an example, etc. Electron-deficient compounds are those that don't have enough electrons to completely fill the octet of the central atom. For each pair of bonded atoms to form a conventional electron-pair bond, these compounds are deficient in electrons. Electron-deficient compounds can be found in polymorphic forms to make up for their lack of electrons. Compounds with less than 8 electrons in their valence shells, such as B2H6, AlH3, etc., are considered to be electron-deficient.
To know more about electron rich please refer: https://brainly.com/question/20388963
#SPJ4
Related Questions
Oxidation state of the iodine (I) in IO3– and chlorine (Cl) in ClO–?
Answers
Answer: The oxidation state of chlorine (Cl) in ClO– is +1.
Explanation:
To determine the oxidation state of iodine (I) in IO3– and chlorine (Cl) in ClO–, we can use the oxidation state rules.
For IO3– (iodate ion):
The sum of the oxidation states for all atoms in a polyatomic ion equals the charge of the ion. In this case, the charge is -1.
Oxygen typically has an oxidation state of -2.
There are three oxygen atoms in the iodate ion.
Let x be the oxidation state of iodine (I). Then, we can write the equation:
x + 3(-2) = -1
x - 6 = -1
x = +5
The oxidation state of iodine (I) in IO3– is +5.
For ClO– (hypochlorite ion):
The sum of the oxidation states for all atoms in a polyatomic ion equals the charge of the ion. In this case, the charge is -1.
Oxygen typically has an oxidation state of -2.
Let y be the oxidation state of chlorine (Cl). Then, we can write the equation:
y + (-2) = -1
y - 2 = -1
y = +1
The oxidation state of chlorine (Cl) in ClO– is +1.
7.0×107 ÷ 2.0×104
turn into a proper scientific notation. PLS HELP
Answers
The expression 7.0x\(10^7\) ÷ 2.0x\(10^4\) can be expressed in proper scientific notation as 3.5x10^3.
To express the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) in proper scientific notation, we need to perform the division and adjust the result to the appropriate format.
Dividing the numbers, we get:
7.0x\(10^7\) ÷ 2.0x\(10^4\)= 3.5x\(10^{(7-4)\)= 3.5x\(10^3\)
The result of the division is 3.5, and we adjust the exponent by subtracting the exponent of the divisor from the exponent of the dividend (7 - 4 = 3).
Therefore, the proper scientific notation representation of the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) is 3.5x\(10^3\).
Scientific notation is a way to express numbers using a coefficient (in this case, 3.5) multiplied by a power of 10 (in this case, 10^3). It allows for more concise representation of very large or very small numbers.
In this case, the division resulted in a number that is smaller than the dividend and has a positive exponent, indicating a smaller magnitude compared to the original numbers. The coefficient represents the significant digits of the result, while the power of 10 represents the scale or magnitude of the number.
For more such questions on scientific notation visit:
https://brainly.com/question/28468914
#SPJ8
A sample of gold has a mass of 23.82
Answers
The volume of the gold sample, given the theoretical density and the mass, is 1.23 ml
How to find the volume ?When given the theoretical density of a gold sample and the mass, you can use the formula for density to find the volume of the gold sample.
The volume for density is:
Density = Mass / Volume
Mass = 23. 82 g
Density = 19. 30 g / ml
The volume is:
19.30 = 23 .82 / Volume
Volume x 19. 30 = 23. 82
Volume = 23. 82 / 19. 30
= 1.23 ml
Find out more on volume at https://brainly.com/question/28717703
#SPJ1
The full question is:
A sample of gold has a mass of 23.82 g, given that the theoretical density is 19.30 g/ml, what is the volume of the gold sample?
The concentration of an aqueous solution of NaCl is 15% by mass. How much NaCl is in a 500 gram sample of the solution?
a. 425 grams
b. 750 grams
c. 75 grams
d. 330 grams
Answers
Answer:
To solve this problem, we can use the definition of percent concentration by mass:
percent concentration = (mass of solute ÷ mass of solution) x 100%
We know that the percent concentration of NaCl in the solution is 15% by mass, and we have a 500 gram sample of the solution. Let's assume that the mass of NaCl in the sample is x grams.
Using the percent concentration formula, we can write:
15% = (x ÷ 500) x 100%
Simplifying this equation, we get:
x = (15 ÷ 100) x 500 = 75 grams
Therefore, there are 75 grams of NaCl in a 500 gram sample of the solution.
The correct answer is c. 75 grams.
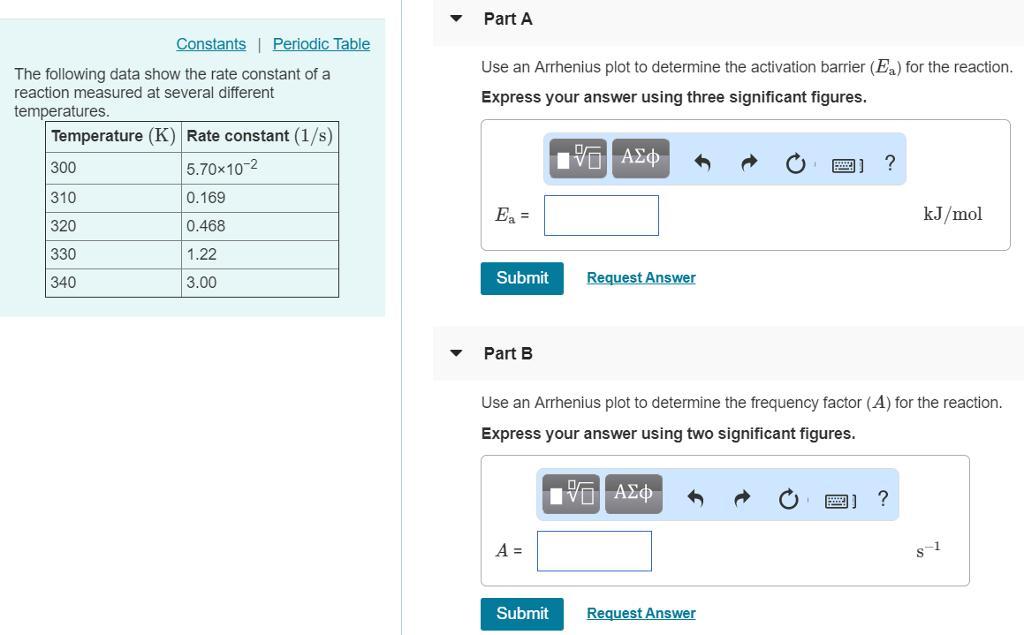
The following data show the rate constant of a reaction measured at several different temperatures. Temperature (K) Rate Constant (1/s) 310 0.194 320 0.554 330 1.48 340 3.74 350 8.97 Part APart complete Use an Arrhenius plot to determine the activation barrier for the reaction. Express your answer using three significant figures. Ea
Answers
Complete Question
The complete question is shown on the first uploaded image
Answer:
Part A
activation barrier for the reaction \(E_a = 84 .0 \ KJ/mol\)
Part B
The frequency plot is \(A = 2.4*10^{13} s^{-1}\)
Explanation:
From the question we are told that
at \(T_1 = 300 \ K\) \(k_1 = 5.70 *10^{-2}\)
and at \(T_2 = 310 \ K\) \(k_2 = 0.169\)
The Arrhenius plot is mathematically represented as
\(ln [\frac{k_2}{k_1} ] = \frac{E_a}{R} [\frac{1}{T_1} - \frac{1}{T_2} ]\)
Where \(E_a\) is the activation barrier for the reaction
R is the gas constant with a value of \(R = 8.314*10^{-3} KJ/mol \cdot K\)
Substituting values
\(ln [\frac{0.169}{6*10^-2{}} ] = \frac{E_a}{8.314*10^{-3}} [\frac{1}{300} - \frac{1}{310} ]\)
=> \(E_a = 84 .0 \ KJ/mol\)
The Arrhenius plot can also be mathematically represented as
\(k = A * e^{-\frac{E_a}{RT} }\)
Here we can use any value of k from the data table with there corresponding temperature let take \(k_2 \ and \ T_2\)
So substituting values
\(0.169 = A e ^{- \frac{84.0}{8.314*10^{-3} * 310} }\)
=> \(A = 2.4*10^{13} s^{-1}\)
An ibuprofen suspension for infants contains 100 mg/5.0 mL suspension. The recommended dose is 10 mg/kg body weight. How many mL of this suspension should be given to an infant weighing 18 lb?
Answers
Answer:
32,8mL
Explanation:
1lb=2.2kg
18lb= 8.2 kg
100mg/5mL=20mg/1mL
164mg=32.8mL
What is The relationship between the electromotive force and the free enthalpy of reaction in a redox reaction?
Answers
Answer:
The EMF and free enthalpy (or Gibbs Free Energy) of a reaction are directly related.
If the free enthalpy of a redox reaction is negative, then the EMF will be negative, indicating that the reaction is spontaneous and will occur without the need for an external source of energy.
If the free enthalpy of the reaction is positive, then the EMF will be positive, indicating that the reaction is not spontaneous and will not occur without the input of energy.
The relationship between free enthalpy and EMF is shown in the following equation:
∆G = -nFE°(cell), where n is the number of electrons transferred, F is the Faraday constant, and E° is the EMF of a cell under standard conditions.
Which of the following are excited in ultraviolet photoelectron spectroscopy?
A. Core electrons
B. Nonvalence electrons
C. X-rays
D. Valence electrons
Answers
Answer:
D. Valance Electrons
Explanation:
3) Tears are produced by the lacrimal glands located:
A) above the lateral canthus of each eye
B) below the medial canthus of each eye
C) below the lateral canthus of each eye
D) above the medial canthus of each eye
E) behind the nasal bones
Answers
3.-4.
A hydraulic pump lifts a solution with a density of 1200 kg/m³ from a
tank to a reservoir, as indicated in the attached diagram. The level difference between
the reservoir and the pump is 15 m, and between the tank and the pump is 2 m. There is
a pressure of 1 bar in the tank and 10 bar in the tank. If the pipes have the
same diameter and the amount of solution to be pumped is 15 kg/s, calculate the
theoretical pumping power.
Z₁ = 2m
Tank
Pi
P2
Deposit
Z2 = 15 m

Answers
The power for the first tank is 2kW and the second tank is 150 kw for the theoretical pumping power lifts a solution with a density of 1200 kg/m³ from a tank to a reservoir.
What is power?Power is the amount of energy in kilowatt which is required to do some work that needs energy known as power it is the multiplication of pressure and the flow of liquid.
Power for the tank = pressure of water × flow of water
Substituting the value,
power = 1 × 2 = 2 kW = P1
power = 10 × 15 = 150 kW = P2
Therefore, a solution with a density of 1200 kg/m³ from a tank to a reservoir power for the first tank is 2kW and for the second tank is 150 kW for the theoretical pumping power lifts.
Learn more about power, here:
https://brainly.com/question/24091709
#SPJ1
Calculate the value deltaG°
Answers
Answer:
ΔG=ΔG0+RTlnQ where Q is the ratio of concentrations (or activities) of the products divided by the reactants. Under standard conditions Q=1 and ΔG=ΔG0 . Under equilibrium conditions, Q=K and ΔG=0 so ΔG0=−RTlnK . Then calculate the ΔH and ΔS for the reaction and the rest of the procedure is unchanged.
Explanation:
A scientific law is different from a scientific theory because it describes something in nature without attempting to explain it.
Answers
Yes, that statement is generally correct. A scientific law is a statement that describes a phenomenon or pattern in nature, often expressed mathematically, without attempting to explain why it occurs. A scientific theory, on the other hand, is a well-substantiated explanation for a set of phenomena, based on empirical evidence and scientific reasoning.
A scientific law summarizes what happens in a particular situation, often in the form of an equation or formula, whereas a scientific theory attempts to explain why it happens.
For example, the law of gravity describes the attraction between masses, but it does not explain why this attraction occurs. In contrast, the theory of general relativity attempts to explain the underlying principles of gravity, including its effects on the curvature of space-time.
It's worth noting that both scientific laws and scientific theories are based on empirical evidence, but they serve different purposes in scientific inquiry. Laws describe what happens in a particular situation, while theories attempt to explain why it happens.
For more question on scientific law click on
https://brainly.com/question/16347879
#SPJ11
Determine the value of Kc for the following reaction, if the equilibrium concentrations are as follows: [N2]eq = 2.66 M, [H2]eq = 0.64 M, [NH3]eq = 3.34 M.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
Answers
The value of Kc for the given reaction is 0.0579 (rounded to four decimal places).
The formula for the equilibrium constant, Kc, of a reaction is given by the ratio of the product of the concentrations of the products raised to their respective stoichiometric coefficients to the product of the concentrations of the reactants raised to their respective stoichiometric coefficients.
The stoichiometric coefficients are the coefficients in the balanced chemical equation.
To determine the value of Kc for the reaction given by the following chemical equation:N2(g) + 3 H2(g) ⇌ 2 NH3(g)
we first need to write the expression for Kc.
The expression for Kc is given by the following formula:Kc = [NH3]² / [N2][H2]³.
We are given the equilibrium concentrations as follows:[N2]eq = 2.66 M[H2]eq = 0.64 M[NH3]eq = 3.34 M
We can substitute these values into the expression for Kc and obtain the following:Kc = (3.34)² / (2.66)(0.64)³ = 0.0579 (rounded to four decimal places).
For more such questions on Kc
https://brainly.com/question/15225808
#SPJ8
A total of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reaction produces 191 g of solution. The reaction caused the temperature of the solution to rise from 21.00 to 24.70 ∘C . What is the enthalpy of this reaction? Assume that no heat is lost to the surroundings or to the coffee cup itself and that the specific heat of the solution is the same as that of pure water. Enter your answer in kilojoules per mole of compound to three significant figures.
Answers
The enthalpy of this reaction is 1.48 kJ/mol.
The formula to calculate heat energy is
Q = m × c × ΔT
ΔT = T₂ - T₁
m = mass (grams)m = 191 gramsc = specific heat capacity (J/g °C)
c pure water = 4.184 J/g °CΔT = temperature change (°C)T₁ = initial temperature = 21.00 °CT₂ = final temperature = 24.70 °CQ = heat energy (J)
ΔT = T₂ - T₁ = 24.70 - 21.00 = 3.70 °C
Q = m × c × ΔT
Q = 191 × 4.184 × 3.70
Q = 2,956.83 J
Q = (2,956.83 ÷ 1,000) kJ
Q = 2.96 kJ
The enthalpy ΔH = Q ÷ n
n = number of moles = 2.00 molQ = heat energy = 2.96 kJΔH = enthalpyΔH = Q ÷ n
ΔH = 2.96 ÷ 2.00
ΔH = 1.48 kJ/mol
Learn more about heat energy here: brainly.com/question/28842664
#SPJ1
True or False: All cells have different basic chemical composition. *
True
False
Answers
identify each unit as belonging to si units or us customary units
Answers
The correct identification of the units is given below:
S.I Units: meter, kilogram and KelvinUS customary units: gallon. mile, pound, degrees FahrenheitWhat is the SI Unit?The SI units and the physical quantities they represent are the meter for length, the second for time, the kilogram for mass, the kelvin for temperature, the candela for light intensity, the ampere for current flow, and the mole for substance amount.
US customary units refer to the system of measures used in the US to measure objects. All other measures are conventional, including the gallon for volume, mile for length, pound for mass, and degree Fahrenheit for temperature.
Read more about SI units here:
https://brainly.com/question/15657466
#SPJ1
identify each unit as belonging to si units or us customary units. gallon. mile. meter. pound. kilogram. degrees farenheight. kelvin.
analyze imagine that a classmate draws an electron dot diagram for a helium atom with two dots. he or she tells you that these dots mean helium atom has two unpaired electrons to have four pairs of valence electrons and become stable. What is wrong with your classmate's argument?
Answers
Answer:
Yo i need this too someone please respond to this
Explanation:
ppppppllllllllllleeeeeeeeeeaaaaaaasssssseeeeeee
How many grams of a 25% (m/m) sodium chloride solution contain 0.250 moles of sodium chloride?
Answers
Approximately 1.167 grams of the 25% (m/m) NaCl solution would contain 0.250 moles of sodium chloride.
To determine the mass of a 25% (m/m) sodium chloride (NaCl) solution containing 0.250 moles of sodium chloride, we need to consider the concentration and molar mass of NaCl.
The percentage (m/m) concentration indicates the mass of solute (NaCl) present per 100 grams of the solution. Therefore, a 25% (m/m) NaCl solution contains 25 grams of NaCl per 100 grams of the solution.
First, we calculate the mass of NaCl in the given solution:
Mass of NaCl = (25% / 100%) x Mass of solution
= (25 / 100) x Mass of solution
Next, we can determine the mass of the solution required to have 0.250 moles of NaCl using the molar mass of NaCl, which is approximately 58.44 g/mol.
Mass of NaCl = Moles x Molar mass
Mass of solution x (25 / 100) = 0.250 moles x 58.44 g/mol
Now, we can solve for the mass of the solution:
Mass of solution = (0.250 moles x 58.44 g/mol) / (25 / 100)
= 1.167 g
for more questions on sodium chloride.
https://brainly.com/question/30460299
#SPJ8
Forensics:
roshni, a forensic scientist, has ruled out several different drugs when testing a substance found in a car. She suspects that the substance is cocsine, what should roshni do next?
1-Perform a confirmatory test
2-Put the substance in a bag, label it, and send it to a DEA office
3-Confer with a colleague about her suspicions.
4-Collect a blood sample and see if it also contains the same substance
Answers
Since she suspects that the substance is cocsine, the thing that she should do next is option 1-Perform a confirmatory test.
How the Evidence Is Collected?The way that Drug evidence is collected is that from any crime scene, evidence is obtained, photographed, packaged, documented as well as been sent for analysis and then the forensic scientist will carry out the Analysis.
Since the evidence has been sent to Roshni, all she has to do is to carry out the confirmatory test to see if the drug is what she suspected.
Therefore, Since she suspects that the substance is cocsine, the thing that she should do next is option 1-Perform a confirmatory test.
Learn more about forensic scientist from
https://brainly.com/question/27111095
#SPJ1
0.276 g of Na2CO3.xH20 was weighed out accurately and dissolved in water. Titration with
0.050 mol cm sulphuric acid required 20.00 cm for neutralisation. Calculate the value of x.
Answers
x in Na₂CO₃.xH₂0 is the number of water molecule that is attached to sodium carbonate called water of crystallization. Therefore the value of x is 10
What is titration?Titration is a technique by which we know the concentration of unknown solution using titration of this solution with solution whose concentration is known. To know the end point we use phenolphthalein as indicator. End point is a point where completion of reaction happen
The balanced equation is
Na₂CO₃ + H₂SO₄ → Na₂SO₄ + H₂O + CO₂
Concentration of H₂SO₄= 0.05 mol/L
Volume of H₂SO₄ = 20 ml = 0.02 L
number of moles of H₂SO₄= 0.05× 0.02 = 0.001mol
number of moles of H₂SO₄ = n(Na2CO3) = 0.001 mol
Molar mass of Na₂CO₃= 106 g/mol
mass of Na₂CO₃ = n× M = 0.001 × 106 = 0.106g
change in mass =∆m = 0.276 – 0.106 = 0.17 g
Molar mass of (H₂O = 18 g/mol
n umber of moles of water = mass÷ Molar mass=0.17 g÷18=0.0094mol
x= number of moles of H₂O÷ number of moles of sodium carbonate
= 0.0094mol÷0.001
=10
Therefore the value of x is 10
To know more about titration, here:
https://brainly.com/question/13307013
#SPJ5
URGENTTTTTTTT
What is the mass of 10.00mL of water at 33℃?

Answers
Answer:
9.93712g
Explanation:
You have 10mL of water at 33 degrees, so you want to look at the density of water at 33 degrees. Then you multiply that density (.993712) by 10 and the mL cancel out and you are left with 9.93712g.
Hope this helps!
72. A 5-mL sample of water has a mass of 5g. What is the density of water?
Answers
Answer: 1 g/ml
Explanation:
5 g / 5ml= 1 g/ml
A 1.50-L bulb containing Ne at 470 torr is connected by a valve to a 2.50-L bulb containing CF4 at 110 torr. The valve between the two bulbs is opened and the two gases mix. The initial gas pressures as known to three significant figures.
(a) What is the partial pressure (torr) of Ne?
(b) What is the partial pressure (torr) of CF4?
(c) What is the total pressure?
(d) What is the mole fraction of Ne?
Answers
(a) Partial pressure of Ne is 470 torr. (b) Partial pressure of of CF₄ is 110 torr. (c) Total pressure is 580 torr. (d) Mole fraction of Ne is 0.621.
(a) The initial pressure of Ne is 470 torr, so the partial pressure of Ne after mixing is also 470 torr.
(b) The initial pressure of CF₄ is 110 torr, so the partial pressure of CF₄ after mixing is also 110 torr.
(c) The total pressure is the sum of the partial pressures of the two gases:
Total pressure = partial pressure of Ne + partial pressure of CF₄
Total pressure = 470 torr + 110 torr
Total pressure = 580 torr
(d) To find the mole fraction of Ne, we need to know the number of moles of Ne and CF₄. We can use the ideal gas law to find the number of moles of each gas:
PV = nRT
n = PV/RT
For Ne:
n = (470 torr x 1.50 L)/(0.0821 L·atm/mol·K x 298 K)
n = 19.25 mol
For CF₄:
n = (110 torr x 2.50 L)/(0.0821 L·atm/mol·K x 298 K)
n = 11.72 mol
The total number of moles is:
nTotal = nNe + nCF₄
nTotal = 19.25 mol + 11.72 mol
nTotal = 30.97 mol
The mole fraction of Ne is:
XNe = nNe/nTotal
XNe = 19.25 mol/30.97 mol
XNe = 0.621
Therefore, the mole fraction of Ne is 0.621.
To know more about partial pressure please refer: https://brainly.com/question/16749630
#SPJ1
When converting from moles into atoms, what number should you put on the top of the conversation factor?

Answers
The number of the atoms that should be the numerator is 6.02 * 10^23 atoms.
What is the conversion factor?We have to note that the conversion factor is the factor that can be used in the conversion of one unit to the other. We have to note that if we are able to change the unit then we can be able to make the conversion.
We have to note that the Avogadro's number is the number of atoms that we can find in one mole of a substance and it has a value of about 6.02 * 10^23 atoms in the atom of magnesium.
Learn more about atoms:https://brainly.com/question/13654549
#SPJ1
A 20.0 g sample of aluminum (specific heat = 0.902) g-1 oC-1) with an initial temperature of 2.5°C is heated with 427 J of
energy. What is the final temperature of the sample?
O 72.3°C
O 23.7°C
0 26.2°C
0 74.8°C
O 24.9°C

Answers
Answer:
T(final) = 26.2°C (3 sig. figs.)
Explanation:
Q = m·c·ΔT
m = mass of sample of interest = 20.0g
c = specific heat of sample of interest = 0.902j·g⁻¹·C⁻¹
ΔT = Temperature change = T(final) - T(initial) = T(f) - 2.5°C
Q = 427 joules
Q = m·c·ΔT => = ΔT = Q/m·c => T(f) = Q/m·c + T(i) = (427j/20.0g·0.902j·g⁻¹·C⁻¹) + 2.5°C = 26.16962306°C (calc. ans.) ≅ 26.2°C (3 sig. figs.)
2C2H2(g) + 502(g) → 4C02(g) + 2H20(g) reaction type?
Answers
Answer:
The answer is combustion.
Which is NOT a compound?
A. silicon dioxide
B. water
C. carbon dioxide gas
D. oxygen gas
Answers
Answer: Oxygen
Explanation: Its found on the periodic table as an element.
what is the final temperature of both the water and the iron?
Answers
Hello!
Answer:
32.8 °C
Hope this helps :)
7.5 L of a gas at 2 ATM and a temperature of 75°C is changed and volume to 3.4 L and a pressure of .5 ATM what is the new temperature
Answers
Answer:
Explanation:
Combined Gas Law
T2= T1P2V2/ (P1V1) = 348.15 X .5 X 3.4/(2 X 7.5) =39.46 K or -233.69C
A local barista serves coffee at 85°C. You add ice to the coffee to cool it to 55°C. Assume that an ice cube is 24 g and -18.5°C. How many ice cubes would you need to add to your 355 mL cup of coffee to bring it to 55°C? The specific heat of ice is 2.05 J/g°C, the specific heat of water is 4.184 J/g°C, and the specific heat of fusion of water is 334 J/g. Remember that an ice cube will need to be warmed to 0°C, will melt, and then the newly melted water will be warmed to 55°C.
A. 1
B. 2
C. 3
D. 4
Answers
To solve this problem, we need to consider the energy required to warm the ice cube from -18.5°C to 0°C, the energy required to melt the ice cube, and the energy required to warm the melted water from 0°C to 55°C. Let's calculate the energy for each step and determine the number of ice cubes needed.
Warming the ice cube to 0°C:
The specific heat capacity of ice is 2.05 J/g°C. The temperature change is from -18.5°C to 0°C. Therefore, the energy required to warm the ice cube can be calculated as:
Energy = mass × specific heat capacity × temperature change
Energy = 24 g × 2.05 J/g°C × (0°C - (-18.5°C))
Energy = 24 g × 2.05 J/g°C × 18.5°C = 899.4 J
Melting the ice cube:
The heat of fusion (specific latent heat of fusion) of water is 334 J/g. We need to determine the energy required to melt the ice cube. The mass of the ice cube is 24 g, so the energy required can be calculated as:
Energy = mass × heat of fusion
Energy = 24 g × 334 J/g = 8016 J
Warming the melted water from 0°C to 55°C:
The specific heat capacity of water is 4.184 J/g°C. We need to determine the energy required to warm the melted water from 0°C to 55°C. The mass of the melted water can be calculated by subtracting the mass of the ice cube that melted from the initial mass of the ice cube (24 g).
Mass of melted water = 24 g - 24 g = 0 g (since all the ice melted)
Therefore, no additional energy is required for this step.
Now, let's add up the energy required for each step to determine the total energy needed to cool the coffee from 85°C to 55°C:
Total energy required = Energy to warm the ice cube to 0°C + Energy to melt the ice cube
Total energy required = 899.4 J + 8016 J = 8915.4 J
Given that each ice cube provides 8915.4 J of energy, we can determine the number of ice cubes needed to cool the coffee.
Energy per ice cube = 8915.4 J
Energy required to cool the coffee = Total energy required = 8915.4 J
The number of ice cubes needed = Energy required to cool the coffee / Energy per ice cube
Number of ice cubes needed = 8915.4 J / 8915.4 J = 1
Therefore, you would need to add 1 ice cube to your 355 mL cup of coffee to bring it to 55°C.
The correct answer is A. 1.