There are certain trends with which you should become very familar (recognizing these trends will save you time!) This part of the question is dedicated to that task.
a) Whenever you see 9 or a multiple of 9 in the integration ratio, which group should you first consider for being responsible for that signal.
b) Whenever you see a quartet and triplet on a spectrum, which group should you first consider for being responsible for those signals?
c) Whenever you see septet and a doublet on a spectrum, which group should you first consider for being responsible for those signals?
d) Whenever you see 6 or a multiple of 6 in the integration ratio, which group should you first consider for being responsible for that signal
e) Whenever you see 3 as the actual number of protons for a given signal, which group should you first consider for being responsible for that signal
Answers
Answer:
Explanation:
The objective of this question is all about identifying the phenomena that holds true for the statement being said in each instance. Let; walk through them.
a) Whenever you see 9 or a multiple of 9 in the integration ratio, which group should you first consider for being responsible for that signal.
( C₄H₉ )Tert. Butyl group
b) Whenever you see a quartet and triplet on a spectrum, which group should you first consider for being responsible for those signals?
(CH₃CH₂) ethyl group
c) Whenever you see septet and a doublet on a spectrum, which group should you first consider for being responsible for those signals?
(CH(CH₃)₂) isoproply group
d) Whenever you see 6 or a multiple of 6 in the integration ratio, which group should you first consider for being responsible for that signal
(CH(CH₃)₂) isoproply group
e) Whenever you see 3 as the actual number of protons for a given signal, which group should you first consider for being responsible for that signal.
CH₃- methyl group
A perfect description showing the explanation of each answers chosen is explained with an aid of diagram below.
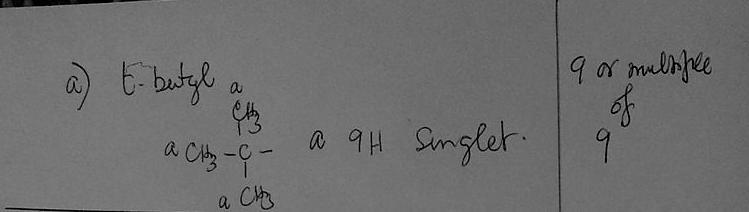
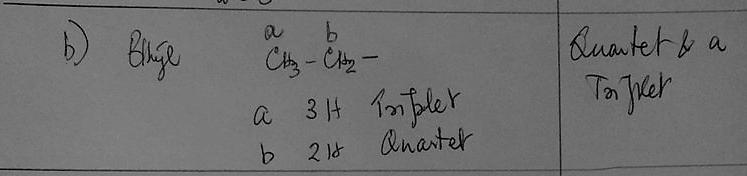
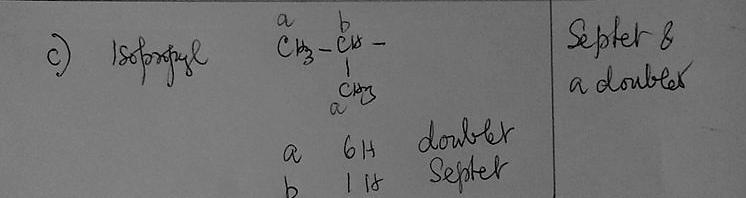
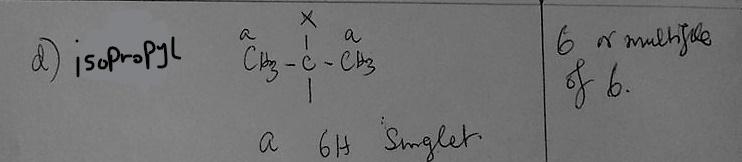
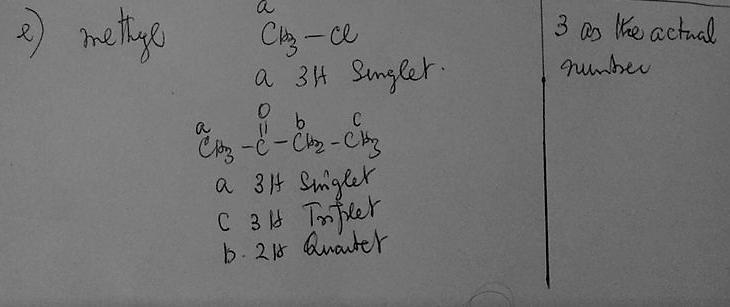
Related Questions
Club soda is an aqueous solution of carbon dioxide. A sample of club soda is titrated with 0.04202M NaOH(aq) according to the reaction equation below:
CO2(aq)+2NaOH(aq)→Na2CO3(aq)
If it takes 32.14 mL of 0.04202M NaOH(aq) to react with a 25.00 mL sample of club soda, what is the concentration of CO2 in club soda (in g/L )?

Answers
The concentration of CO2 in club soda is approximately 1.1964 g/L.
To find the concentration of CO2 in club soda, we need to use the stoichiometry of the reaction and the volume and concentration of the NaOH solution used.
The balanced equation for the reaction is:
CO2(aq) + 2NaOH(aq) → Na2CO3(aq)
From the stoichiometry of the equation, we can see that 1 mole of CO2 reacts with 2 moles of NaOH. Therefore, the moles of CO2 can be calculated using the volume and concentration of NaOH solution used.
Given that 32.14 mL of 0.04202 M NaOH solution was used, we can calculate the moles of NaOH:
moles of NaOH = volume (L) × concentration (M)
moles of NaOH = 32.14 mL × 0.04202 mol/L
moles of NaOH = 0.001351 mol
According to the stoichiometry of the equation, 1 mole of CO2 reacts with 2 moles of NaOH. Therefore, the moles of CO2 can be calculated as:
moles of CO2 = (moles of NaOH) / 2
moles of CO2 = 0.001351 mol / 2
moles of CO2 = 0.0006755 mol
Now, we need to convert the moles of CO2 to grams. The molar mass of CO2 is approximately 44.01 g/mol.
mass of CO2 = moles of CO2 × molar mass of CO2
mass of CO2 = 0.0006755 mol × 44.01 g/mol
mass of CO2 = 0.02979 g
Finally, we need to express the concentration of CO2 in club soda in g/L. We are given that the sample of club soda used is 25.00 mL.
concentration of CO2 = (mass of CO2) / (volume of club soda in L)
concentration of CO2 = 0.02979 g / (25.00 mL × 0.001 L/mL)
concentration of CO2 = 1.1964 g/L
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
2. What causes a current in an electric circuit?
conductor
voltage
ampere
electric charge
Answers
Answer:
electric charge
Explanation:
Using this equation, m1v2=m2v2 , calculate the diluted molarity of 100 mL of a 0.5 M solution when 50 mL of
water has been added.
Answers
The molarity of the diluted solution is 0.33 M
From the question given above, the following data were obtained:
Molarity of stock solution (M₁) = 0. 5 M
Volume of stock solution (V₁) = 100 mL
Volume of diluted solution (V₂) = 100 + 50 = 150 mL
Molarity of diluted solution (M₂) =?The molarity of the diluted solution can be obtained by using the dilution formula as illustrated below:
M₁V₁ = M₂V₂0.5 × 100 = M₂ × 150
50 = M₂ × 150
Divide both side by 150
M₂ = 50 / 150
M₂ = 0.33 MTherefore, the molarity of the diluted solution is 0.33 M
Learn more: https://brainly.com/question/24625656
How many single covalent bonds could an element with the electron
configuration 1s2 2s2 2p5 form?
A-2
B-3
C-1
D-4
Answers
Answer:
1
Explanation:
I would say 1 because that configuration has 7 valence electrons. The goal of the atom is to gain a full octet so it just needs to gain 1 more electron to get the full octet. To get the one electron to fill the valence shell it would only need to have 1 covalent bond.
I hope this helps. Let me know if anything is unclear.
A single covalent bond could be formed by an element with the electron configuration 1s2 2s2 2p5 in the form C.1.
How do electron configurations work?An electron configuration is a description of the positions of electrons around a nucleus. The symbols used to represent the electron configuration start with the shell number (n), then the type of orbital, and finally the superscript indicates how many electrons are in the orbital. As previously stated, each neutral atom contains a specific number of electrons.I would answer 1 because there are 7 valence electrons in this configuration. Because that is what the atom desires, it only requires one more electron to complete its octet. For the single electron to fill the valence shell, only one covalent link would be required.To learn more about electron configurations refer to:
https://brainly.com/question/26084288
#SPJ2
How many molecules of water are there in 8.050 x 103 grams of water?
Answers
Answer:
2.71 × 10²⁰ molecules
Explanation:
Given data:
Mass of water = 8.050 × 10³ g
Number of molecules = ?
Solution;
First of all we will calculate the number of moles of water,
Number of moles = mass/molar mass
Number of moles = 8.050 × 10³ g / 18 g/mol
Number of moles = 0.45 × 10³ mol
1 mole contain 6.022 × 10²³ molecules,
0.45 × 10³ mol × 6.022 × 10²³ molecules / 1 mol
2.71 × 10²⁰ molecules
The number 6.022 × 10²³ is called Avogadro number.
It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance.
DONT SKIPP What type of bond will occur between lithium and bromine?
A.
these atoms will not bond
B.
metallic
C.
ionic
D.
covalent
Answers
Number of atoms in 2.56 moles of He
Answers
1.54 × 10²⁴ atoms He
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
MolesAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
[Given] 2.56 moles He
[Solve] atoms He
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
[DA] Set up: \(\displaystyle 2.56 \ mol \ He(\frac{6.022 \cdot 10^{23} \ atoms \ He}{1 \ mol \ He})\)[DA] Multiply [Cancel out units]: \(\displaystyle 1.54163 \cdot 10^{24} \ atoms \ He\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
1.54163 × 10²⁴ atoms He ≈ 1.54 × 10²⁴ atoms He
Chemical formula for barium chromate
Answers
Answer:
Ba + Cr + O₄
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
Think of an example in which chemical and physical weathering affects a rock
Answers
Answer:
chemical-Acid rain eating away at the rock
Physical-Dust particles eroding the rock overtime in the wind
Explanation:
Volcanic eruptions inflation

Answers
The volcanic eruptions inflation are listed below.
What is volcanic eruption ?
Lava and gas are occasionally discharged explosively from a volcano during a volcanic eruption. When newly erupted lava cascades down a volcano's flanks, it is known as a "glowing avalanche" and is the most deadly sort of eruption. Temperatures of up to 1,200 degrees Fahrenheit can be reached and they can move swiftly.
What is eruption?
The silica content and gas content of the magma play a major role in determining the features of the four different types of eruptions. These include the Hawaiian, Strombolian, Vulcanian, and Plinian eruptions, in order of increasing explosiveness.
The earth surface often expands when magma builds up in an underground reservoir prior to an eruption (named inflation). Similarly, as magma exits the reservoir with the potential to erupt, the land above the reservoir sinks (named deflation).
Therefore, volcanic eruptions inflation are listed above.
Learn more about volcanic eruption from the given link.
https://brainly.com/question/25121802
#SPJ1
What is the distinguishing characteristic of invertebrates?
Group of answer choices
Specialized tissues and organ systems
The lack of a centralized brain
The lack of a spinal column
Being an insect
Answers
The lack of a spinal column is the distinguishing characteristic of invertebrates.
What is an Invertebrate?These are organisms which lack a backbone(vertebral column) while vertebrates on the other hand have it.
This is why option C was chosen as the most appropriate choice in this scenario.
Read more about Invertebrate here https://brainly.com/question/21332744
#SPJ1
Three 15.0 mL acid samples - 0.10 M HA, 0.10M HB, and 0.10 M H2C - are all titrated with 0.100 M NaOH.If HA is a weak acid, HB is a strong acid, and H2C is a diprotic acid, which statement is true of all three titrations?
All three titrations have the same final pH.
All three titrations require the same volume of NaOH to reach the first equivalence point.
All three titrations have the same pH at the first equivalence point.
All three titrations have the same initial pH.
Answers
All three titrations require the same volume of NaOH to reach the first equivalence point.
The type of acid used in the titration determines the pH of the result: a strong acid results in a pH of 7 at the titration's equivalent. Equivalence point for a weak acid is influenced by the type of conjugate base and weak acid's beginning pH. The type of the acid is another factor for a diprotic acid.
Thus:
The initial pH of all three titrations is the same, as is the pH at the first equivalence point in all three titrations. Moreover, the final pH of all three titrations is the same. of the three are FALSE.
Thus:
To reach the first equivalence point, all three titrations need the same volume of NaOH.
Learn more about equivalence point here:
https://brainly.com/question/29999744
#SPJ4
If HA is the weak acid, HB is the strong acid, and H₂C is a diprotic acid, the statement is true for the titrations is All three titrations require the same volume of NaOH to reach the first equivalence point.
The pH of the titration is depend on the nature of acid. here the the HA is the weak acid and the HB is the strong acid. the H₂C is the diprotic acid. the diprotic acid means the acid which contains the two hydrogen atoms and will ionized in the water.
Thus, the from all the statements the true one is that All three titrations require the same volume of NaOH to reach the first equivalence point.
To learn more about titration here
https://brainly.com/question/17199543
#SPJ4
Relate application of compounds to their properties and to their
bonding and structure
Answers
Answer:Answer:
Properties of ionic compounds
Ionic compounds have regular structures, called giant ionic lattices. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions between the oppositely charged ions. The structure and bonding of ionic compounds explain their properties.
Energy must be transferred to a substance to make it melt or boil. This energy overcomes the strong electrostatic forces of attraction which act in all directions between the oppositely charged ions:
some forces are overcome during melting
all remaining forces are overcome during boiling
The more energy needed, the higher the melting point or boiling point. Since the electrostatic forces of attraction between oppositely charged ions are strong, their melting and boiling points are high.
Explanation
Ionic compounds are held together by electrostatic forces between the oppositely charged ions. These forces are usually referred to as ionic bonding. As the ionic lattice contains such a large number of ions, a lot of energy is needed to overcome this ionic bonding so ionic compounds have high melting and boiling points.
The strength of the ionic bonds depends on the charge on the ions. Ions with higher charge will have stronger forces between them, so will need more energy in order to overcome these forces.
Explanation:
500.0 mL of a 0.205 M solution of LiBr is diluted to 700.0 mL. What is the new concentration of the solution?
Answers
Answer:
0.146 M
Explanation:
Use v1s1 = v2s2
here, v1 = 500 mL, v2 = 700 mL, s1 = 0.205 M & s2 = new concentration
Determine the number of protons (p) and electrons (e) in Mg2.
A) 10 p, 12 e
B) 12 p, 12 e
C) 12 p. 10 e
D) 12 p. 14 e
E) 10 p, 10 e
Answers
What is the phase of water at 1.0 atm and 50°C?
Water
(liquid)
Water vapor
(gas)
100
Temperature (°C)
Pressure (atm)
1-
0.5-
0.25-
0
Ice
(solid)
-10
OA. Liquid and gas
OB. Liquid
OC. Solid
D. Gas
0-
0.01
Answers
The given temperature of water is 50 degrees Celsius, hence, we can conclude that the phase of the given water is liquid phase.
What is phase of water?
Water can exist in three different phases, namely;
Liquid phase - usually between 0 to 100 degrees CelsiusSolid phase - below 0 degrees CelsiusVapor phase or gaseous phase - usually above 100 degrees CelsiusTherefore, if the given temperature of water is 50 degrees Celsius, hence, we can conclude that the phase of the given water is liquid phase.
Learn more about water phase here: https://brainly.com/question/1612862
#SPJ1
Which of the following would make it easier for a plant to do photosynthesis?
O Colorful flowers
O Very large leaves.
Very tall stem.
O Short roots
Answers
What is an electromagnetic wave?
Answers
Answer:
An electromagnetic wave is a wave that is created as a result of vibrations between an electric field and a magnetic field.
Explanation:
Hope this helps!!
What happens to dew or frost each day after the sun rises?
Answers
Evaporation causes the dew to vanish when the temperature of the atmosphere rises with the rising of the sun.
What is evaporation ?A kind of vaporization called evaporation takes place on the surface of a liquid as it transitions into the gas phase. When humidity impacts the rate of evaporation of water, for example, a high concentration of the evaporating material in the surrounding gas considerably slows down evaporation.
Similar to how perspiration evaporates from your body on a hot day to cool you down, dew evaporates as it cools the plant. This lessens heat exhaustion in extremely hot settings. Some species, particularly desert plants, are capable of directly absorbing water via their leaves.
Thus, Evaporation causes the dew to vanish when the temperature of the atmosphere rises with the rising of the sun.
To learn more about evaporation, follow the link;
https://brainly.com/question/5019199
#SPJ1
You are an intermediate product of an industrial process which intends to separate iron from its ore. A well known iron ore is hematite. Which of these ores does not contain iron?
Goethite
Malachite
Siderite
Limonite
Answers
Answer:
Malachite
Explanation:
Malachite is a copper carbonate hydroxide mineral, with the equation Cu2CO3(OH)2. This dark, green-joined mineral solidifies in the monoclinic precious stone framework, and frequently shapes botryoidal, sinewy, or stalagmitic masses, in cracks and profound, underground spaces, where the water table and aqueous liquids give the way to synthetic precipitation. So, the answer is malachite. Best of Luck!
Question 3
you've researched and analyzed. now it's time to present your findings! compose a three-paragraph newspaper article about the development. include and expand upon your responses to the questions in questions 1 and 2. be sure to use quotes from your sources and cite them properly. make sure your article uses correct spelling and grammer, topic statements, an introduction, a conclusion, and smooth transitions.
Answers
Answer:
free
Explanation:
hi
Answer:
I think it in edmentum
Explanation:
Please help me, im studying for finals and i need an answer to this question! Will mark brainliest for the best answers!w

Answers
Answer:
the generation of electricity and other energy jointly, especially the utilization of the steam left over from electricity generation to produce heat.
I hope it helps!! Have a nice day
Answer:
Cogeneration r is the use of a heat engine or power station to generate electricity and useful heat at the same time.
was added mixtus 31-6g of Potassium pitrate solid was 120 am ³ of water in a plastic beaker. The was stirred gently and the following results. Inital temperature =21-5°C Final temperature =17-0°C a) Calculate the enthalpy change for the reaction. (Density = Iglam³, C² = 4.2jg-1k-1
Answers
According to the problem the enthalpy change for the reaction is 742 J.
What is enthalpy?Enthalpy is a thermodynamic quantity that measures the total heat content of a system. It is the sum of the internal energy of the system plus the product of its pressure and volume. In simple terms, it is the total amount of energy contained in a system, including the energy of its components and any energy exchanged with the environment.
The enthalpy change (ΔH) for the reaction can be calculated using the equation:
ΔH = (mass of solution x C² x ΔT) / (density of solution)
Where:
mass of solution = 31.6 g
C² = 4.2 J g-1K-1
ΔT = (21.5 – 17.0) °C = 4.5 °C
density of solution = 1.00 g/cm³
Therefore, ΔH = (31.6 g x 4.2 J g-1K-1 x 4.5 °C) / (1.00 g/cm³)
= 742 J
The enthalpy change for the reaction is 742 J.
To learn more about enthalpy
https://brainly.com/question/14047927
#SPJ1
Fatty foods become rancid due to the process of
Answers
Answer:
Fatty foods become rancid due to the process of rancidity
Why doesn't the outer electron on the potassium atom experience the full charge of the protons in the nucleus (+19)?

Answers
Answer:
There are 18 electrons shielding it from the nucleus
Explanation:
The shielding effect is the reduction in the effective nuclear charge on an electron, due to repulsion by the inner electrons in the atom.
Potassium has 19 electrons, 18 of which are in the inner shells of the atom. These 18 electrons "shield" the outermost electron from feeling the effect of the nuclear charge. The magnitude of effective nuclear charge felt by an electron as a result of screening by other inner electrons is given by Slater's rules.
48 g of Aluminum will produce how much heat (in kJ) for this reaction?
Use the following reaction:
2 Al + 3 CuSO4 → 3 Cu + Al2(SO4)3 ∆H = -680 kJ
Round your answer to a positive whole number and do not write the unit.
Answers
The heat produced from 48 g Aluminium = -578
Further explanationGiven
Reaction
2 Al + 3 CuSO4 → 3 Cu + Al2(SO4)3 ∆H = -680 kJ
48 g of Aluminum
Required
Heat released
Solution
mol of Aluminium = mass : Ar Al
mol = 48 g : 27 g/mol
mol = 1.78
From the reaction, heat released for 2 moles Al, so for 1.78 Al, the heat released :
\(\tt \dfrac{1.7}{2}\times -680~kJ=-578~kJ\)
What is 5.678 + 82.5, to the correct significant figures?
Answers
5.678 plus 82.5 equals 88.178. The solution should be rounded to the least amount of decimal places in any of the numbers, which is one decimal place in 82.5 as both values contain three significant figures.
Therefore, 88.2 is the final response, rounded to the proper significant digits.
Significant digits.The number of meaningful digits in a number is referred to as a significant figure. They are crucial for computations and measurements in science since they show how precise a measurement was.
The least precise measurement or value used in the calculation usually determines the number of significant numbers in a measurement or calculation.
The final result should be rounded to the same number of decimal places or significant figures as the calculation's least exact number when adding or subtracting values with differing amounts of decimal places or significant figures.
In this instance, 5.678 contains four significant figures, but 82.5 has three. The final result should therefore be rounded to three significant digits.
learn more about significant figures here
https://brainly.com/question/24491627
#SPJ1
Brainiest if you are correct and this is a Test I just need help on the last one.

Answers
Answer:
Explanation
i k the ansewer
Help pls i really need help

Answers
Answer: I think it's 1
Explanation: Potential energy means stored energy . In Position 1 it's not moving the energy is being stored. Hope that helps.