Answers
The term middle ages was coined by a roman pope to describe the period between the end of the roman empire and the spread of Christianity is false.
The given statement is false. the term was coined in the seventeenth century that described the period of the between the end of the western roman empire. the seventeenth century will rise the states of Europeans. the Christianity began to spread in the century that is first century. the rome was exited in the mid of the fifteenth century.
Thus, the given statement is false that the term middle ages was coined by a roman pope that describe the period between the end of the roman empire and the spread of Christianity.
To learn roman empire here
https://brainly.com/question/11968092
#SPJ4
Related Questions
A gas absorbs 21.39 kJ of energy while expanding against 0.276 atm from a volume of 0.0432 L to 1.876 L. What is the energy change of the gas?
Answers
Answer:
Q = 21.896kJ
Explanation:
Q = ?
∇U = 21.39kJ
W = ?
W = P∇V
W = P (V2 - V1)
W = 0.276 × (1.876 - 0.04232)
W = 0.276 × 1.83368
W = 0.5060J
Q = ∇U + W
Q = 21.39 + 0.5060
Q = 21.896kJ
The energy change corresponds to the work done by the system
Answer:
\(\Delta E=21.34kJ\)
Explanation:
Hello,
In this case, we should apply the first law of thermodynamics to compute the energy change:
\(\Delta E=Q-W\)
Thus, with the given volume change we compute the corresponding work in kJ:
\(W=P\Delta V=0.276atm*(1.876L-0.0432L)*\frac{101.325kPa}{1atm}*\frac{1m^3}{1000L}=0.0513kJ\)
Then, we compute the energy change:
\(\Delta E=21.39kJ-0.0512kJ\\\\\Delta E=21.34kJ\)
Best regards.
Calculate number of molecules in 5.3 g of sodium carbonate Na2CO3
Answers
We have a sample of 5.3 g of Na₂CO₃. Before finding the number of molecules we have to find the number of moles. When we are given the mass of a sample and we have to find the number of moles in that sample, we usually use the molar mass. The molar mass can be calculated using the atomic masses of the elements that are in the compound. If we look fot the atomic masses, we find:
Na: 22.99 amu C: 12.01 amu O: 16.00 amu
Since one molecule of Na₂CO₃ has 2 atoms of Na, 1 atom of C and 3 atoms of O, the molar mass of Na₂CO₃ is:
molar mass of Na₂CO₃ = 2 *22.99 + 1 *12.01 + 3 *16.00
molar mass of Na₂CO₃ = 105.99 g/mol
Now that we found the molar mass of Na₂CO₃, we can find the number of moles that we have in the 5.3 g sample of it.
number of moles of Na₂CO₃ = 5.3 g / (105.99 g/mol)
number of moles of Na₂CO₃ = 0.050 moles
In one mol of molecules we have 6.022 *10^23 molecules. using that relationship we can find the answer to our problem:
1 mol of molecules = 6.022 *10^23 molecules
number of molecules = 0.050 moles * 6.022 * 10^23 molecules/1 mol
number of molecules = 3.0 * 10^23 molecules
Answer: there are 3.0 * 10^23 molecules in 5.3 g of sodium carbonate.
what is the vapour pressure at 298K of a 20% solution of KNO3 by mass. Vapour pressure of water at 298K is 3.2kPa
Answers
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

Examine the substances listed. Identify the substance(s) that represents a single element.
SUBSTANCE: soil, copper pipe, water. CO2*
soil
copper pipe
water
CO2
Answers
Answer:
the answer is copper pipe
Which of the following has kinetic energy?
Wind blowing at 3 km/h
A fully stretched elastic band
A boulder sitting on top of a hill
An unused battery
Answers
Answer:
wind blowing
Explanation:
"Wind is energy in motion—kinetic energy"
Answer: Wind Blowing
Explanation:
How many liters of chlorine are needed to react with 31.2
Answers
Answer:
Explanation: all the answer for the question is that link
Please Help!
About to fail my class for this
Answers
IUPAC (International Union of Pure and Applied Chemistry) naming is also known as systematic naming.
It is a set of rules and guidelines used to name chemical compounds systematically. It provides a standardized method for naming organic and inorganic compounds based on their molecular structure and composition.
The longest continuous carbon chain in the compound is identified as the parent chain.
The IUPAC name of the given compounds are:
2-methyl,2-hexene4-ethyl, 3,5-dimethyl nonane4-methyl, 2-heptene5-propyl decane2-methyl butane2-methyl, 2-penteneLearn more about IUPAC nomenclature, here:
https://brainly.com/question/14379357
#SPJ1
which element has the highest ionization energy?B, Al, Ga, In
Answers
Answer:
BORON
Ionization energy decreases down the group and going from left to right the period,
Luckily you have the elements from the same group that is Group III A also called boron family,
The position of Elements in this group are
Boron (B)Aluminium (Al)Gallium (Ga)Indium(In)ThalliumNihoiumso keeping rules in mind the first element in the group has highest I.E. that is boron
Select the TWO statements that are true about the amount and types of energy that are visible when each of the light bulbs are shining.
A. The incandescent light bulb does not create as much as much light energy as the fluorescent light bulb.
B. When the fluorescent light bulb is lit, more light energy is visible than thermal energy.
C. The thermal energy is stored in the incandescent light bulb before it is transformed into light energy.
D. More thermal energy is visible when the fluorescent light bulb is lit.

Answers
A. The incandescent light bulb does not create as much as much light energy as the fluorescent light bulb.
Why do you think fluorescent bulbs are more energy-efficient than incandescent ones?Incandescent light bulbs have the drawback of wasting a lot of electricity due to heat. All the energy used to create heat is a waste because heat does not produce light, and the light bulb's intended function is to produce light. Thus, incandescent lamps are quite ineffective.
Why does a fluorescent tube produce light that is brighter and uses less energy than an incandescent lamp?Mercury vapor is excited by an electric current in the gas to produce short-wave ultraviolet light, which illuminates a phosphor coating inside the lamp. Compared to an incandescent lamp, a fluorescent lamp is far more efficient at converting electrical energy into usable light.
To know more about the fluorescent light visit:
https://brainly.com/question/8979272
#SPJ1
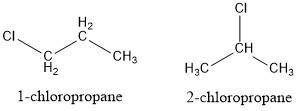
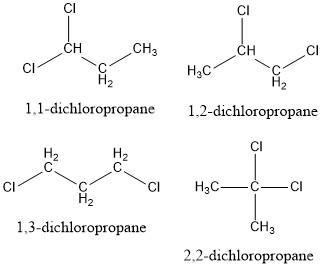
Write the structural and condensed formulas as well as the names for all isomers of C3H7Cl and C3H6Cl2.?
Answers
Answer:
\(C_3H_7Cl\) = Two structures
\(C_3H_6Cl_2\) = Four structures
Explanation:
We must remember that in an isomer we have the same molecular formula but different structures. Thus, for the molecule \(C_3H_7Cl\) we can draw a linear structure of 3 carbons and change the position of the chlorine atom, obtaining two different structures.
For the molecule \(C_3H_6Cl_2\), we can use similar logic. Place a chain of 3 carbons and change the position of the chlorine atoms in such a way that for this formula we will have 4 different structures.
See figures 1 and 2 for further explanations.
All isotopes of an element have a different number of ____.
a.
orbital shells
b.
neutrons
c.
protons
d.
electrons
e.
atoms
Answers
Protons and electrons have the same amount and atoms.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
a solid block of substance is 74.0 cm by 55.0 cm by 29.0 cm and it weighs 625 kg. Determine the density. Would it float in water? The density of water is 1 g/cm^3. Show your work.
Answers
Explanation:
It is given that,
Dimension of a solid block of substance is 74.0 cm by 55.0 cm by 29.0 cm
Mass of solid block is 625 kg or 625000 grams
We need to find the density of solution. The mass per unit its volume is called density.
\(\rho=\dfrac{m}{V}\\\\\rho=\dfrac{625000}{74\times 5\times 29}\\\\\rho=58.24\ g/cm^3\)
So, the density of solid block is \(58.24\ g/cm^3\). The density of water is \(1\ g/cm^3\). The density of block is more than water. Hence, it will sink.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
1)Grignard reagent when reacted with methanol will yield A) ethanol (B) secondary alcohols (C) tertiary alcohols (D ropanol (E) primary alcohol
Answers
When the reaction of Grignard reagent reacted with methanol will yield a tertiary alcohol. Therefore, Option C tertiary alcohol is correct.
Contains a carbon-metal link, Grignard reagents are chemicals used in catalysis. They generally result from the anhydrous reaction of magnesium metal with an alkyl or aryl halide. Because of their high reactivity, Grignard reagents frequently act as nucleophiles in organic reactions.
An alkyl group from a Grignard reagent binds to the oxygen atom of methanol (CH3OH) when it interacts with the methanol, breaking the carbon-metal connection. A precursor alkoxide is created as a result. The equivalent alcohol is then produced by protonating the intermediate alkoxide.
The reaction of a Grignard reagent with methanol leads to the formation of a tertiary alcohol.
Learn more about reagents here:
https://brainly.com/question/29729676
You find yourself in a room with dark gray walls. Medeleev’s image says, “This element is essential for plant life to thrive and is found in heavy clay minerals and the ash from your campfire.” What element are these walls made from?
Answers
Answer:
juvn hgf jb ujvi i junk food sux
Explanation:
Hello I need help with this question

Answers
Deleted answer. ......
QUESTION 6 Consider the following reaction between the diatomic and monatomic forms of iodine: I2 (g) <-> 2I (g) When 0.095 M I2 is initially placed in a previously empty container and sealed, the system slowly reaches equilibrium. When equilibrium is reached, it is found that there is an equilibrium concentration of 0.0055 M of the monatomic form of iodine. Calculate the (unitless) equilibrium constant Kc. Round your answer to two sig figs, and express it in scientific notation.
Answers
Answer: The equilibrium constant is \(3.3\times 10^{-4}\)
Explanation:
Initial concentration of \(I_2\) = 0.095 M
The given balanced equilibrium reaction is,
\(I_2(g)\rightleftharpoons 2I(g)\)
Initial conc. 0.095 M 0 M
At eqm. conc. (0.095-x) M (2x) M
Given : 2x = 0.0055
x = 0.00275
The expression for equilibrium constant for this reaction will be,
\(K_c=\frac{[l]^2}{[I_2]}\)
Now put all the given values in this expression, we get :
\(K_c=\frac{(0.0055)^2}{(0.095-0.00275)}\)
\(K_c=\frac{(0.0055)^2}{0.09225}=0.00033\)
Thus the equilibrium constant is \(3.3\times 10^{-4}\)
According to the collision theory, when can a chemical reaction occur? (3 points)
A. When enough activation energy is added to correct the orientation of the particle collisions
B. When reactants collide with enough energy to intersect their valence shells and form new bonds
C. When reactants collide with enough mass to form new bonds and break apart the reactants
D. When the proper catalyst is added to break the chemical bonds in the reactants
Answers
The presence of a catalyst, as mentioned in option D, can provide an alternative reaction pathway with lower activation energy, but it is not a necessary condition for a chemical reaction to occur according to the collision theory. Option D
According to the collision theory, a chemical reaction can occur under the following conditions:
When reactants collide: For a chemical reaction to occur, the reactant particles must come into contact with each other. Collisions between reactant molecules are necessary to initiate the reaction. This point aligns with option B, as reactants colliding with enough energy is an essential aspect of the collision theory.
When collisions have enough energy: Not all collisions between reactant particles result in a chemical reaction. According to the collision theory, a reaction can occur only if the colliding particles have sufficient energy to overcome the activation energy barrier.
This means that the collision should provide enough energy to break the existing bonds in the reactant molecules, allowing new bonds to form. This point supports option B, as collisions with enough energy to intersect valence shells and form new bonds are necessary for a reaction to take place.
When the proper orientation is achieved: In addition to having enough energy, the reactant molecules must collide with the correct orientation for a chemical reaction to occur. The collision should bring the reactive parts of the molecules into contact with each other to allow bond formation or breaking.
This point supports option A, as the correct orientation of the reactant particles during collisions is crucial for the reaction to proceed successfully.
Option D
For more such question on catalyst visit:
https://brainly.com/question/21598276
#SPJ8
An element has 2 stable isotopes. One has 13 amu and 1.07% abundant . The second has 12 amu and 98.93% abundant. What is the average atomic mass of the element
Answers
The average atomic mass of the element is 12.0107 amu.
To calculate the average atomic mass of the element in question, we can use the following formula:
average atomic mass = (mass of isotope 1 x abundance of isotope 1) + (mass of isotope 2 x abundance of isotope 2)
where "mass of isotope 1" is the mass of the first stable isotope (13 amu in this case), "abundance of isotope 1" is the percentage of that isotope in the element (1.07% in this case), "mass of isotope 2" is the mass of the second stable isotope (12 amu in this case), and "abundance of isotope 2" is the percentage of that isotope in the element (98.93% in this case).
Substituting the given values in the formula, we get:
average atomic mass = (13 amu x 1.07%) + (12 amu x 98.93%)
average atomic mass = (0.1391 amu) + (11.8716 amu)
average atomic mass = 12.0107 amu
Therefore, the average atomic mass of the element is 12.0107 amu.
This means that on average, one atom of this element weighs 12.0107 atomic mass units (amu), which is slightly heavier than the most abundant isotope (12 amu) due to the presence of the less abundant isotope (13 amu). This concept is important in chemistry because the mass of atoms plays a crucial role in determining their chemical and physical properties. The knowledge of the average atomic mass of an element is important in a wide range of applications, including analytical chemistry, geochemistry, and nuclear physics.
Know more about atomic mass here:
https://brainly.com/question/3187640
#SPJ11
For the reaction C+2H₂ → CH4, how many moles of carbon are needed to make 128.1 grams of
methane, CH4?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer
as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your
answer incorrect.:
Hydrogen: 1
Carbon: 12
Answers
The term mole concept is used here to determine the moles of carbon. The moles of carbon required to make 128.1 g of CH₄ is 8 mol.
What is a mole?One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles = Given mass / Molar mass
The number of moles of 'C' required is:
128.1 g CH₄ / 1 × 1 mol CH₄ / 16 g CH₄ × 1 mol C / 1 mol CH₄ = 8.00 mol C.
Thus the number of moles of 'C' required is 8 mol.
To know more about mole concept, visit;
https://brainly.com/question/19730733
#SPJ1
PLEASE ANSWER QUICKLY!!
100 NaNO3
90
Solute per 100 g of H₂O (g)
0,80
NH,CI
70 KNO3
60
50
40
30
20
10
0
0 10 20 30 40 50 60 70 80 90 100
Temperature (°C)
KCIO3
60 g KNO3 has
been added to
100 g H₂O at
30 °C. What
type of solution
is this?
A. unsaturated
B. saturated
C. supersaturated

Answers
If 60 grams of the substance are added to 100 g of water, the solution can be categorized as supersaturated.
How saturated is this solution?The graph shows the number of grams that can be dissolved in 100 grams of water at different temperatures. In general, solubility increases with temperature.
According to the graph, at a temperature of 30°C, it is possible to dissolve a total of 48 to 49 grams of \(KNO_{3}\). This information implies that if we add 60 grams at this temperature not all the substance would be dissolved, and therefore the solution would be supersaturated.
Learn more about solubility in https://brainly.com/question/31493083
#SPJ1
When 1600 joules of heat is added to a sample of solid copper (Cu), the temperature rises from 15.0 C to 30.0 C. the specific heat of solid copper is 0.385, how many grams of copper are in the sample.
Answers
Taking into account the definition of sensible heat, the mass of copper in the sample is 277.06 grams.
Definition of sensible heatWhen heat added or removed from a substance causes a temperature change in it without affecting its molecular structure (physical state), it is called sensible heat.
The expression that allows to calculate heat exchanges is:
Q = c× m× ΔT
where:
Q is the heat exchanged by a body of mass m.c is the specific heat substance.ΔT is the temperature variation.Mass of copper in the sampleIn this case, you know:
Q= 1600 Jc= 0.385 J/gCm= ?ΔT= Tfinal - Tinitial= 30 C - 15 C= 15 CReplacing in the definition of sensible heat:
1600 J = 0.385 J/gC× m× 15 C
Solving:
1600 J = 5.775 J/g× m
1600 J÷ 5.775 J/g= m
277.06 g= m
Finally, the mass of copper in this case is 277.06 grams.
Learn more about sensible heat:
brainly.com/question/16744669
brainly.com/question/15500310
#SPJ1
2.62 Predict the chemical formulas of the compounds formed by the following pairs of ions: (a) Cr3+ and Br, (b) Fe3+ and O2, (c) Hg22+ and CO2, (d) Ca2+ and CIO3, (e) NH4+ and PO³
Answers
Answer:
(a) Cr3+ and Br- will form CrBr3 (chromium(III) bromide)
(b) Fe3+ and O2- will form Fe2O3 (iron(III) oxide)
(c) Hg22+ and CO32- will form Hg2CO3 (mercury(I) carbonate)
(d) Ca2+ and ClO3- will form Ca(ClO3)2 (calcium chlorate)
(e) NH4+ and PO43- will form (NH4)3PO4 (ammonium phosphate)
Explanation:
chatGPT
The chemical formulas for the compounds formed by the given pairs of ions are: (a) CrBr3, (b) Fe2O3, (c) Hg2(CO3)2, (d) Ca(ClO3)2, and (e) (NH4)3PO4.
Explanation:(a) Cr3+ and Br- : In order to form a neutral compound, the charges of the ions must balance. The charge of Cr3+ is 3+ and the charge of Br- is 1-. To balance the charges, we need three Br- ions for every Cr3+ ion. Therefore, the chemical formula is CrBr3.
(b) Fe3+ and O2- : The charge of Fe3+ is 3+ and the charge of O2- is 2-. To balance the charges, we need two O2- ions for every Fe3+ ion. Therefore, the chemical formula is Fe2O3.
(c) Hg22+ and CO2- : The charge of Hg22+ is 2+ and the charge of CO2- is 2-. The charges are already balanced, so no extra ions are needed. Therefore, the chemical formula is Hg2(CO3)2.
(d) Ca2+ and ClO3- : The charge of Ca2+ is 2+ and the charge of ClO3- is 1-. To balance the charges, we need two ClO3- ions for every Ca2+ ion. Therefore, the chemical formula is Ca(ClO3)2.
(e) NH4+ and PO3- : The charge of NH4+ is 1+ and the charge of PO3- is 3-. To balance the charges, we need three NH4+ ions for every PO3- ion. Therefore, the chemical formula is (NH4)3PO4.
Learn more about Chemical Formulas here:https://brainly.com/question/36379566
#SPJ3
Determine the mass in grams of 3.07 moles of glucose (C6H12O6)
Answers
Mass in grams of 3.07 moles of glucose is 553.3 g (rounded to three significant figures).
What is Mass?
Mass is a fundamental physical property of matter that quantifies the amount of matter in an object. It is a measure of the resistance of an object to acceleration when a force is applied to it. In other words, mass is the amount of substance in an object, and it determines how much gravity will affect that object.
To determine the mass in grams of 3.07 moles of glucose (C6H12O6), we need to first calculate the molar mass of glucose, which is the sum of the atomic masses of all the atoms in its chemical formula:
Molar mass of C6H12O6 = 6(12.01 g/mol) + 12(1.01 g/mol) + 6(16.00 g/mol) = 180.18 g/mol
Next, we can use the following conversion factor to convert moles to grams:
mass (g) = number of moles x molar mass (g/mol)
mass = 3.07 mol x 180.18 g/mol = 553.3 g
Learn more about Mass from given link
https://brainly.com/question/86444
#SPJ1
from kinatic point of view explain the change from solid to liquied based on the effect of change of tempreture.
Answers
Answer:
Temperature affects the kinetic energy in a gas the most, followed by a comparable liquid, and then a comparable solid. The higher the temperature, the higher the average kinetic energy, but the magnitude of this difference depends on the amount of motion intrinsically present within these phases.
Explanation:
Liquids have more kinetic energy than solids. When a substance increases in temperature, heat is being added, and its particles are gaining kinetic energy. Because of their close proximity to one another, liquid and solid particles experience intermolecular forces. These forces keep particles close together.
A 5.0 g sample of metal was heated from 10°C to 40. °C. It absorbed 43.7 J of energy as heat. What is the specific heat of this piece of metal?
Answers
Answer:
Balls
Explanation:
Cuz
Read the statement. Magnesium atoms have 12 electrons, with the configuration 2-8-2. If the first five ionization energies of magnesium are shown, which ionization energy would show a sudden sharp increase?
Answers
The electronic configuration of magnesium is:
1s² 2s² 2p⁶ 3s² = [Ne] 3s²
This means that for a magnesium atom, in order to have its outermost orbital full, the easiest way would be to lose the two outermost electrons. This is seen in its relatively low first two ionization energies. The third ionization energy is several times higher because the ion would move from a stable form to an highly unstable form. (Mg⁺² → Mg⁺³ + e⁻).
Sodium only has one electron in its outermost orbital, so its first ionization energy would be several times lower than the second.
Question 4 of 25aFor a bond to be covalent, the electronegativity difference between the atomsmust be:OA. zero.B. less than 1.7.OC. less than 0.5.D. over 2.

Answers
Answer:
B. Less than 1.7.
Explanation:
A covalent bond can be polar o non polar, but if the electronegativity difference between the atoms is less than 1.7 will be covalent polar and it will be covalent nonpolar if it is less that 0.5.