Answers
Answer:
Your answer will be 34.5 g
Explanation:
The molar mass of aluminum (Al) is 26.98 g/mol. In the formula above, replace the values. This results in 34.5 g of aluminum (Al) being required.
Related Questions
what is the mass of 3.40 x 10^35 formula units of KCl??
Answers
The mass of 3.40 x 10^35 formula units of KCL is 4.20 * 10^13.
What is meant by the formula unit?The empirical formula of an ionic or covalent network solid compound that is used as a separate entity for stoichiometric calculations is known as a formula unit in chemistry. It is also the ionic compound with the lowest whole number ratio of ions.
As we know,1 mole = 6.02 x 1023 molecules.
1 = (1 mole)/(6.02x1023 molecules)
So, (3.40x10^35 molecules KCL)*(1 mole KCL)/(6.02x1023 molecules KCL)
5.647 *10^11 moles Na2SO4
Molar mass of KCL = 74.55 g/mol
Mass of KCL will be = 5.647 *10^11 * 74.55
Hence, mass of KCL = 4.20 * 10^13
To know more about formula unit, refer
brainly.com/question/24712181
#SPJ1
What is the molarity of the solution formed by mixing 0.2 moles of NaOH with enough water to make 0.15 L of solution?
Answers
Answer: 0.5
Explanation:
Which of these is NOT true about sandy soil?
A) It's gritty to the touch.
B) It crumbles, even when wet.
C) It's made of large particles.
D) It supports many kinds of plants.
Answers
What is The relationship between the electromotive force and the free enthalpy of reaction in a redox reaction?
Answers
Answer:
The EMF and free enthalpy (or Gibbs Free Energy) of a reaction are directly related.
If the free enthalpy of a redox reaction is negative, then the EMF will be negative, indicating that the reaction is spontaneous and will occur without the need for an external source of energy.
If the free enthalpy of the reaction is positive, then the EMF will be positive, indicating that the reaction is not spontaneous and will not occur without the input of energy.
The relationship between free enthalpy and EMF is shown in the following equation:
∆G = -nFE°(cell), where n is the number of electrons transferred, F is the Faraday constant, and E° is the EMF of a cell under standard conditions.
how does a battery get its power?
Answers
Answer:
Batteries get its power from the stored energy inside it- chemical energy- which is then converted into electrical energy. The flow of the electrons allow the electric current to do work.
Explanation:
Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu.
Answers
Answer:
32.057 amu
Explanation:
\(\text{Average atomic mass = Mass}\times \text{abundances}\)
\(M=95\%\ \text{of}\ 31.972 +0.76\%\ \text{of}\ 32.971 +4.22\%\ \text{of}\ 33.967 \\\\M=\dfrac{95}{100}\times 31.972 +\dfrac{0.76}{100}\ \times \ 32.971 +\dfrac{4.22}{100} \times \ 33.967\\\\M=32.057\ \text{amu}\)
So, the average atomic mass of sulfur is 32.057 amu.
The average atomic mass of sulfur is 32.06 amu if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu.
Sulfur is formed by 3 isotopes: 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu.
The average atomic mass (am) is a weighted average that takes into account the mass and the abundance of each isotope.
\(am = \frac{\Sigma m_i \times ab_i }{100} = \frac{31.972amu \times 95.00 + 32.971amu \times 0.76 + 33.967amu \times 4.22}{100} = 32.06 amu\)
The average atomic mass of sulfur is 32.06 amu if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu.
Learn more: https://brainly.com/question/13753702

A student investigates the reaction between sodium hydroxide solution and dilute sulphuric acid. He does a titration to find the concentration of sulphuric acid. Describe four changes that the student could make to improve his plan
Answers
To know the end point we use phenolphthalein as indicator. End point is a point where completion of reaction happen. Therefore, to improve the plan, student can follow the given techniques.
What is titration?Titration is a technique by which we know the concentration of unknown solution using titration of this solution with solution whose concentration is known.
The balanced equation is
NaCl + H\(_2\)SO\(_4\)\(\rightarrow\) HCl + Na\(_2\)SO\(_4\)
The changes that the student could make to improve his plan are
Use the indicator to know the end point.
solution should be kept in clean beaker.
Solution should be standardised.
Burette should be clamped perfectly.
Therefore, to improve the plan, student can follow the above techniques.
To know more about titration, here:
https://brainly.com/question/13307013
#SPJ1
The pictures show butter and water in solid states. The melting point of each substance is shown.
Students put solid butter and solid water on a table. The air temperature around the table is 20°C.
Answers
Students put solid butter and solid water on a table. The air temperature around the table is 20°C. After two hours, The butter will remain a solid, and the water will become a liquid. Therefore, option B is correct.
What do you mean by states of matter ?A state of matter is one of the various forms that matter can take. In everyday life, four states of matter are visible: solid, liquid, gas, and plasma.
On a table, students placed solid butter and solid water. The temperature of the air around the table is 20°C. The butter will remain solid after two hours, while the water will become liquid.
Thus, option B is correct.
To learn more about the states of matter, follow the link;
https://brainly.com/question/29069107
#SPJ9
Your question is incomplete, most probably your question was
The pictures show butter and water in solid states. The melting point of each substance is shown.
Students put solid butter and solid water on a table. The air temperature around the table is 20°C.
A. The butter and water will both become liquids.
B. The butter will remain a solid, and the water will become a liquid.
C. The butter and water will both remain solids.
D. The butter will become a liquid, and the water will remain a solid.
What is the liquid substance use in the laboratory for dissolving dry mortar on floor flies
Answers
The liquid substance used in the laboratory for dissolving dry mortar on floor flies is hydrochloric acid.
What is hydrochloric acid?Hydrochloric acid is a strong acid that can dissolve many materials, including dry mortar.
Hydrochloric acid also known as muriatic acid or sulfuric acid, are commonly used to dissolve hardened mortar or concrete residues.
To use hydrochloric acid to dissolve dry mortar, you will need to mix the acid with water in a ratio of 1 part acd to 10 parts water.
You should then apply the mixture to the dry mortar using a brush or spray botle.
Find more exercises on Hydrochloric acid;
https://brainly.com/question/24784580
#SPJ1
How many grams of propane contains the same number of carbon atoms as those in 1.0g C2H5OH ?
Answers
Answer:
Exercises Example 6. How Many Moles Of Carbon Atoms And How Many Carbon Atoms Are Contained In 1.0 G Of C,H,OH
Explanation:
The mass of the propane that would have the same number of carbon atoms is 0.63 g.
What is the number of the carbon atoms?We know that we can be able to obtain the number of the carbon atoms by the use of the simple stoichiometry of the reaction as we have it in the question that we have been asked here.
We can see that we have the molar mass of the ethanol as we can see as 46 g/mol. We have to obtain the number of moles of the ethanol that we have in the compound as follows;
Number of moles = 1 g/ 46 g/mol = 0.022 moles
Number of the carbon atoms that we have is; 0.022 moles * 2 * 6.02 * 10^23 = 2.6 * 10^22 atoms
Now the number of the grams of the propane that we have is;
2.6 * 10^22 = x * 3 * 6.02 * 10^23
Where x is the number of moles present
x = 2.6 * 10^22 / 1.806 * 10^24
x = 1.44 * 10^-2 moles
x = 0.0144 moles
Given that the molar mass of the propane is 44 g/mol
mass = 0.0144 moles * 44 g/mol
Mass = 0.63 g
Learn more about number of atoms:https://brainly.com/question/14190064
#SPJ1
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
What coefficient of 02 should be added so the number of atoms of oxygen is conserved on both sides of the reaction equation
Answers
Answer:
5
Explanation:
Please help due tofay

Answers
Answer:
I only know the answer to the second one, The bond that occurs between two metal atoms is a metallic bond
Molecular geometry of NCI 4 +
Answers
Answer:
blah blah blah blah i need the points
Explanation:
sorry bro
buna, am nevoie de un site de facut proiecte
Answers
Answer:
translation to English please
Helppppp pleaseeee xxxxxx
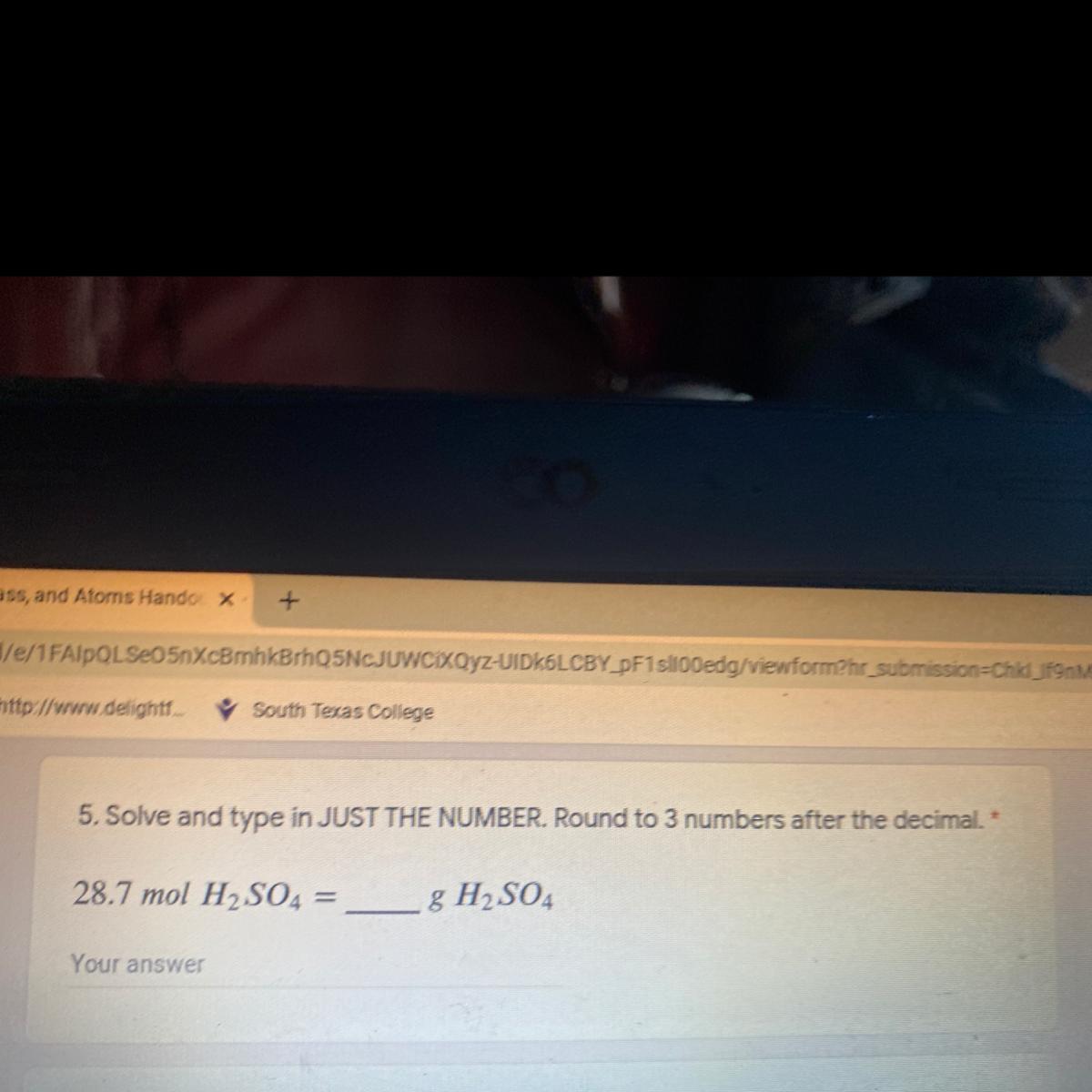
Answers
Answer:
2812.6 g of H₂SO₄
Explanation:
From the question given above, the following data were obtained:
Mole of H₂SO₄ = 28.7 moles
Mass of H₂SO₄ =?
Next, we shall determine the molar mass of H₂SO₄. This can be obtained as follow:
Molar mass of H₂SO₄ = (1×2) + 32 + (16×4)
= 2 + 32 + 64
= 98 g/mol
Finally, we shall determine the mass of H₂SO₄. This can be obtained as follow:
Mole of H₂SO₄ = 28.7 moles
Molar mass of H₂SO₄ =
Mass of H₂SO₄ =?
Mole = mass / Molar mass
28.7 = Mass of H₂SO₄ / 98
Cross multiply
Mass of H₂SO₄ = 28.7 × 98
Mass of H₂SO₄ = 2812.6 g
Thus, 28.7 mole of H₂SO₄ is equivalent to 2812.6 g of H₂SO₄
Chlorophyll allows plants to carry out photosynthesis. There are two forms of chlorophyll:
chlorophyll al ( C55H7205N, Mg) and chlorophyll 6 ( C55H7006N, Mg). What is the
difference in molar mass between these two forms?
Answers
Answer:
Chlorophyll-a/b-carotenoid complexes, known as light harvesting complexes, protect photosynthetic system from strong light. Chlorophylls are insoluble in water due to the absence of polar groups.
Explanation:
Hope this helps :)
985.2 moles of nitrogen, how many moles of ammonia can produce?
Answers
Answer:
985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
Explanation:
The balanced chemical equation for the production of ammonia from nitrogen is:
N2 + 3H2 → 2NH3
From the balanced equation, we can see that 1 mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia.
So, to determine how many moles of ammonia can be produced from 985.2 moles of nitrogen, we need to use the mole ratio from the balanced chemical equation as follows:
985.2 moles N2 x (2 moles NH3 / 1 mole N2) = 1970.4 moles NH3
Therefore, 985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
HELP FAST 100 PTS Calculate the amount of heat needed to raise the temperature of 96 g of water vapor from 124 °C to 158 °C. must provide explanation
Answers
Answer:
\(\huge\boxed{\sf Q = 13.7\ Joules}\)
Explanation:
Given Data:
Mass = m = 96 g = 0.096 kg
\(T_1\) = 124 °C
\(T_2\) = 158 °C
Change in Temp. = ΔT = 158 - 124 = 34 °C
Specific Heat Constant = c = 4.186 J/g °C
Required:
Specific Heat Capacity = Q = ?
Formula:
Q = mcΔT
Solution:
Q = (0.096)(4.186)(34)
Q = 13.7 Joules
\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807Answer:
\(\Large \boxed{\sf 13600 \ J}\)
Explanation:
Use formula
\(\displaystyle \sf Heat \ (J)=mass \ (g) \times specific \ heat \ capacity \ (Jg^{-1}\°C^{-1}) \times change \ in \ temperature \ (\°C)\)
Specific heat capacity of water is 4.18 J/(g °C)
Substitute the values in formula and evaluate
\(\displaystyle \sf Heat \ (J)=96 \ g \times 4.18 \ Jg^{-1}\°C^{-1} \times (158\°C-124 \°C)\)
\(\displaystyle Q=96 \times 4.18 \times (158-124 )=13643.52\)
A ramp is at an angle of elevation of 15° . The distance from the top of the ramp to the ground is 20 feet. What is the length of the ramp?
Answers
Answer:
Length of ramp = 77.27ft
Explanation:
Step 1 - Sketch a diagram
Make a line about 15 deg. above the horizontal x-axis. Draw the horizontal and vertical components to form a right triangle. See image if you don’t understand.
Step 2 - Label your diagram
Your triangle creates a 15 deg. angle with the x-axis and we know the vertical distance from the top of the ramp to the ground is 20 ft.
Step 3 - Use trig to make an equation and solve
SOH CAH TOA
I’m this problem we know the angle, know “opposite, and we are solving for the hypotenuse of the triangle. Therefore, it would be easiest to use SOH or sin(theta)= (opp./hyp.) Plug in the numbers and solve for the hypotenuse.
All work is shown in the attached image.

Under what conditions is n2o3 No gas + n02 gas
spontaneous?
Answers
The reaction is spontaneous under conditions of low pressure and high temperature
What is a spontaneous reaction?We can say that a reaction is spontaneous when we know that the reaction is able to go on on its own. This implies that there is a mnimum energy that is required for the reaction to proceed.
The reaction is thus a sort of a self propagating system that goes on freely of its own accord.. We can see that what is going on here is the decomposition of the nitrogen V oxide gas as shown.
Learn more about decomposition reaction:https://brainly.com/question/16987748
#SPJ1
Describe the motion of molecules in these two
substances when the ice cube is placed on the
radiator.
Answers
Answer:
When in direct contact, the fast-moving molecules in the radiator collide with the slow-moving molecules in the ice cube. Kinetic energy is transferred from the molecules in the radiator to the molecules in the ice cube, causing a slowdown of the radiator molecules and an acceleration of the ice molecules.
Explanation:
Answer
when in direct contact, the fast-moving molecules in the radiator collide with the slow-moving molecules in the ice cube. Kinetic energy is transferred from the molecules in the radiator to the molecules in the ice cube, causing a slowdown of the radiator molecules and an acceleration of the ice molecules.
Explanation:
20. (01.08 MC)
Alchemy is a branch of ancient knowledge that states that all matter is composed of air, fire, earth, ar
Which of these best explains whether alchemy is a science or pseudoscience? (4 points)
It is a science because all matter is known to be made of elements.
it is a science because air, fire, earth, and water all are forms of matter.
It is pseudoscience because all matter is known to be made of atoms.
It is pseudoscience because ancient knowledge is mystical yet reliable.
Answers
It is pseudoscience because each metal is known to be a unique element alchemy is a science or pseudoscience.
What is a matter in chemistry?Matter is anything that occupies space and has mass; in other respects, matter is the "thing" that the cosmos is made of. The fundamental constituents of all stuff are called elements. These are not changed into other elements by conventional chemical processes and have distinct chemical and physical properties.
What does matter consist of?Matter can be solid, liquid, or gaseous on Earth. Solid particles, fluids, and gases are supported by the exceptionally small building components known as atoms and molecules. A solid's component particles are attracted to one another strongly. They are close to one another and vibrate en position without passing each other.
To know more about Matter visit:
https://brainly.com/question/28945834
#SPJ13
The complete question is -
Alchemy is a branch of ancient knowledge that states that all matter is composed of air, fire, earth. Which of these best explains whether alchemy is a science or pseudoscience?
A-It is a science because all matter is known to be made of elements.
B-It is a science because air, fire, earth, and water all are forms of matter.
C-It is pseudoscience because all matter is known to be made of atoms.
D-It is pseudoscience because ancient knowledge is mystical yet reliable.
E-It is pseudoscience because each metal is known to be a unique element.
QUESTION 5
What is a mixture?
O Contains one kind of particle and they DO NOT keep thier own identity and properties.
O Contains one kind of particle.
O Contains MORE than one kind of particle and they keep their own identity and properties.
O Contains MORE than one kind of particle and they DO NOT keep their own identity and properties.
Answers
Answer:
C
Explanation:
Cccccccccccccccccccc
Answer:
The third option.O contains more than one kind of particles anddd they keep their own identify and properties because the definition of mixture is a material made up of 2 or more different substances which are physically combined and identities are retained
Question 3(Multiple Choice Worth 3 points) (01.03 LC) What is potential energy? The energy of change O The energy of position or composition O The energy of mass or volume O The energy of motion
Answers
Potential energy is often referred to as the energy of position or composition. The energy that an object has due to its tension, electric charge, or relative immobility in space is known as potential energy. Potential energy is among the two basic forms of energy.
William Rankine, a Scottish engineer and physicist, coined the phrase "potential energy" in the 19th century. Potential energy comes in a variety of forms, each linked to a particular kind of force. It is the power imparted to an object by its position in relation to other objects. Learn more about potential energy in this article, which includes a definition, several categories, and examples.
The force acting on the two objects affects the formula for potential energy. The following is the formula for gravitational force:
W = m×g×h = mgh
Where,
m is the mass in kilogramsg is the acceleration due to gravityh is the height in metersPotential Energy Unit
In terms of units, kinetic energy and gravitational potential energy are equivalent: kg m2 / s2.
The unit used to measure all energy is the joule, which has the same units as kg m2 / s2 (J).
Learn more about Potential Energy here
https://brainly.com/question/24284560
#SPJ9
which compoud is propanic acid
Answers
Answer:
Explanation:
Propionic acid has chemical formula of C₃H₆O₂ , and smells somewhat unpleasant (like body odour).
When one of the metals in an alloy is mercury the alloy is called a amalgam B sodium amalgam C zinc amalgam D all the above?; What is an alloy of mercury with some other metal generally known as?; Which element is used for making amalgam?; Is amalgam an alloy of mercury?
Answers
When one of the metals in an alloy is mercury the alloy is called a amalgam.
What is alloy?Alloy is defined as the compound which is produced on mixing metals and non-metals. This type of combination is mainly generated in the way to increase those properties of the metals which are absent or they are lacking and addition of non-metals provides it to them. For example: Addition of silver and gold produces an alloy which is known as white gold.
What is Amalgam?Alloy of mercury with other metals or non-metals is known as Amalgam. Amalgam which we prepare can be in liquid, solid or in paste form depending on the property of mercury. Only the alloys of mercury with metals such as tin, zinc, silver, and copper are used as amalgams.
Thus, we concluded that when one of the metals in an alloy is mercury the alloy is called a amalgam.
learn more about Alloy:
https://brainly.com/question/1759694
#SPJ4
What is the volume of 115 g of H2O vapor at 121 °C and 1.00 atm?
Answers
200 L is roughly the volume of 115 g of water vapor at 121 °C and 1.00 atm.
Calculation-
Ideal Gas Law:
PV = nRT
First, we need to convert the given temperature of 121 °C to Kelvin:
T = 121 °C + 273.15 = 394.15 K
n = m/M = 115 g / 18.015 g/mol = 6.386 mol
Now we can rearrange the Ideal Gas Law to solve for volume:
V = nRT/P
V = (6.386 mol)(0.0821 L·atm/mol·K)(394.15 K)/(1.00 atm)
V = 200 L
How may the ideal gas law equation be changed?To find R, the gas constant, the ideal gas law can be modified. R=PiViniTi under initial conditions, and R=PVT under final conditions. Both expressions are equal to each other because they both equal R. In situations where one or more of the variables are constant, this equation is frequently utilized.
to know more about volume here;
brainly.com/question/1578538
#SPJ4
Given: glow is a find the indicated measure.
Answers
Answer:
is there supposed to be some type of image or ...
Explanation: