Rust is what type of Change?
Physical Change
Chemical Change
Answers
Answer:
Chemical change
Explanation:
Rust cannot be done without using chemicals.
Answer:
Option B.) Chemical Change
Related Questions
¿Cual es porcentaje de una solucion que contiene 10g de sal disueltos en 500 mL de solución?
Answers
Answer:
0.02 g/ml
Explanation:
si dividimos 10 con 500, es decir, 10/500, obtenemos 0.02. Las unidades serán g / ml.
Concentration (M)
Reaction: G F
What does the
graph tell us about
this reaction at
equilibrium?
Time (sec)
A. The reaction is reactant favored (K<1).
B. The reaction is product favored (K>1).
C. The reaction has equal concentrations of reactants
and products.

Answers
Answer:
i think its a if not sorry i have it in a test right now
Explanation:
Aluminum bromide and chlorine gas react to
form aluminum chloride and bromine gas.

Answers
Answer:
ok
Explanation:
If there are 3 moles of Pb, how many particles of Pb3N2 are there in the balanced equation? *
Answers
In the balanced equation for the reaction of Pb with N2, 3 moles of Pb would react with 2 moles of N2 to form 6 moles of Pb3N2. Since 1 mole of a substance is equal to 6.02x1023 particles, 3 moles of Pb would be equal to 1.81x1024 particles of Pb.
Similarly, 2 moles of N2 would be equal to 1.21x1024 particles of N2. When these two react to form Pb3N2, 6 moles of Pb3N2 would be formed, which is equal to 3.63x1024 particles of Pb3N2. Thus, if there are 3 moles of Pb, then there are 3.63x1024 particles of Pb3N2.
Molecules and atoms are the building blocks of all matter in the universe. A mole is a unit of measurement used to quantify the amount of a substance present in a given sample. It is defined as the amount of substance that contains the same number of particles as 12 grams of Carbon-12.
Moles are used to calculate the number of particles present in a given amount of a substance, as the number of particles in a mole of a substance is always the same. This allows us to easily calculate the number of particles present in any given amount of a substance.
In chemistry, the balanced equation of a reaction is used to calculate the amount of each reactant and product present in the reaction. Knowing the number of moles of each substance present in the reaction allows us to calculate the number of particles present in each substance as well.
Know more about Carbon-12 here
https://brainly.com/question/7666959#
#SPJ11
What two of the following organisms are secondary consumers in this food web?
Answers
Secondary consumers are organisms that primarily feed on herbivores or other primary consumers.
They occupy the next trophic level above the primary consumers in a food web. They obtain energy by consuming the primary consumers and play an important role in regulating the population of herbivores.
Examples of commonly observed secondary consumers include:
Carnivorous mammals: Animals such as wolves, lions, and tigers that feed on herbivores like deer, zebras, or gazelles.
Birds of prey: Species like eagles, hawks, and owls that consume small mammals, reptiles, or other birds.
Carnivorous fish: Fish like pike, barracuda, or bass that prey on smaller fish or aquatic invertebrates.
Predatory insects: Insects such as spiders, mantises, or dragonflies that feed on other insects, including herbivorous insects.
In a specific food web, the identification of secondary consumers would depend on the specific organisms present and their feeding interactions. It would be necessary to analyze the trophic relationships among the organisms in the food web to determine the secondary consumers accurately.
For more such questions on Secondary consumers visit:
https://brainly.com/question/28631974
#SPJ8
PLS HELPPPP ME!!!!
What term do we use when the moon's light is going away and the moon seems
to be "shrinking" each day? Hint: Karate Kid: "Wax on wane off.”
HELPP PLSSSSS
Answers
How many grams are in 9.05 x 1023 atoms of silicon
Answers
Answer:
42.2075 grams
Explanation:
Define [Fluid compressibility, Solution-gas/liquid ratio, Fluid FVF, Fluid densities, and Fluid viscosities], write their equations, symbols, units \& correlations. (25-points)
Answers
1. Fluid compressibility (C): Fluid compressibility refers to the measure of how much a fluid's volume changes in response to a change in pressure.
2. Solution-gas/liquid ratio (SGLR): The solution-gas/liquid ratio represents the volume of gas dissolved in a given volume of liquid at a specific pressure and temperature.
3. Fluid formation volume factor (FVF): The fluid formation volume factor represents the ratio of the volume of a fluid at reservoir conditions (pressure and temperature) to its volume at surface conditions.
4. Fluid densities (ρ): Fluid densities refer to the mass per unit volume of a fluid.
5. Fluid viscosities (μ): Fluid viscosities represent the measure of a fluid's resistance to flow.
1. Equation: C = -1/V * dV/dP
Symbol: C
Unit: 1/Pascal (Pa^-1)
Correlation: The compressibility of fluids can vary depending on the fluid type. For ideal gases, the compressibility is inversely proportional to pressure.
2.Equation: SGLR = V_gas / V_liquid
Symbol: SGLR
Unit: Volumetric ratio (e.g., scf/bbl)
Correlation: The solution-gas/liquid ratio is influenced by the pressure and temperature conditions, as well as the composition of the fluid.
3. Equation: FVF = V_reservoir / V_surface
Symbol: FVF
Unit: Volumetric ratio (e.g., bbl/STB)
Correlation: The fluid formation volume factor depends on the composition and properties of the fluid, as well as the reservoir conditions.
4. Equation: ρ = m / V
Symbol: ρ
Unit: Mass per unit volume (e.g., kg/m^3)
Correlation: Fluid densities can vary depending on the type and composition of the fluid. For example, water has a density of approximately 1000 kg/m^3.
5. Equation: No single equation; viscosity is measured experimentally using viscometers.
Symbol: μ
Unit: Pascal-second (Pa·s) or centipoise (cP)
Correlation: The viscosity of a fluid is influenced by temperature and pressure. Different fluids exhibit different viscosities, ranging from low-viscosity fluids like water to high-viscosity fluids like heavy oil.
To know more about Fluid formation volume factor (FVF)
https://brainly.com/question/31458735
#SPJ11
which dot and cross diagram is incorrect?

Answers
The dot structure that can be shown to be incorrect is the dot structure that has been shown by option A
What is the dot structure?The Lewis structure is based on the concept that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration similar to that of a noble gas. In the Lewis structure, the valence electrons of the atoms are represented as dots around the symbol of the atom.
We can see that in the dot structure that is in option A the both atoms are coming from the hydrogen atoms and shoud not be differently marked.
Learn more about Lewis structure:https://brainly.com/question/32988499
#SPJ1
The half-life for Carbon-14 is 5614 years. An ancient piece of cloth is found to contain ¼ of its original Carbon-14. How old is the cloth? Describe or show in detail how you solved this.
Answers
Answer:
To determine the age of the ancient cloth, we can use the concept of radioactive decay and the half-life of Carbon-14.
Carbon-14 is a radioactive isotope of carbon, which decays over time into nitrogen-14 through beta decay. The half-life of Carbon-14 is 5614 years, which means that after 5614 years, half of the original amount of Carbon-14 in a sample will have decayed.
In this case, the cloth contains only ¼ of its original Carbon-14. This means that three half-lives have passed since the cloth was first created, as each half-life reduces the amount of Carbon-14 by half.
To determine the age of the cloth, we can use the following formula:
N = N0(1/2)^t/T
where N is the current amount of Carbon-14 in the cloth, N0 is the original amount of Carbon-14 in the cloth, t is the time that has passed, and T is the half-life of Carbon-14.
We know that N = ¼ N0, and T = 5614 years. Plugging these values into the formula, we get:
¼ N0 = N0(1/2)^(3/T)
Solving for t, we get:
t = (3/T) * log(2)
Substituting in T = 5614 years, we get:
t = (3/5614) * log(2) ≈ 1,684 years
Therefore, the cloth is approximately 1,684 years old.
In summary, we can use the concept of radioactive decay and the half-life of Carbon-14 to determine the age of the ancient cloth. By knowing the current amount of Carbon-14 in the cloth, we can calculate the time that has passed since it was first created using a simple formula. In this case, the cloth is approximately 1,684 years old.
What is the major product formed upon treatment of (R) 2-bromohexane with sodium cyanide? a. (R) 2-cyano hexane b. (S) 2-cyanohexane c. 1-hexene d. 2-hexene
Answers
The major product formed upon treatment of (R) 2-bromohexane with sodium cyanide is (S) 2-cyanohexane. The correct option is b.
When (R) 2-bromohexane is treated with sodium cyanide (NaCN), a nucleophilic substitution reaction takes place. The nucleophile, cyanide ion (CN⁻), attacks the electrophilic carbon of the bromoalkane, resulting in the replacement of the bromine atom with a cyanide group.
Since the starting compound, (R) 2-bromohexane, has a chirality center at the second carbon atom, the reaction proceeds with inversion of configuration due to the nucleophilic attack at the backside of the molecule. As a result, the major product formed is the (S) enantiomer of 2-cyanohexane.
The reason for this inversion of configuration is due to the SN2 (substitution nucleophilic bimolecular) mechanism, where the nucleophile attacks the carbon atom bearing the leaving group from the opposite side, leading to a complete reversal of stereochemistry.
Therefore, the correct answer is b. (S) 2-cyanohexane, which represents the major product obtained from the reaction between (R) 2-bromohexane and sodium cyanide.
To know more about nucleophilic substitution refer here:
https://brainly.com/question/32657850#
#SPJ11
Calcite (the main mineral in limestone) is made of calcium carbonate (caco3). dolomite, a related mineral, is made of magnesium carbonate (mgco3). what happens if a geologist drips a small amount of vinegar (acetic acid) onto a sample of dolomite? there is no way to predict what will happen. fizzing will occur because carbon dioxide is produced. no reaction will occur because dolomite contains no calcium.
Answers
If a geologist drips a small amount of vinegar (acetic acid) onto a sample of dolomite, fizzing will occur because carbon dioxide is produced.
Why Carbon dioxide show effervescence?Effervescence observes whenever any gas tried to escape out from an aqueous solution, that's why CO₂ show effervescence.
Reaction between dolomite and acetic acid will be represented as:
MgCO₃ + 2CH₃COOH → CO₂ + H₂O + Mg(CH₃COO)₂
From the above reaction it is clear that carbon dixide gas is produced by showing the effervescence and fizzing behavior.
Hence, option (2) is correct.
To know more about dolomite, visit the below link:
https://brainly.com/question/4945288
Answer:
B
Explanation:
on edge2022
What volume is occupied by 0.25 moles of nitrogen gas at 273 K and 1.50 atm.
Answers
Answer:
3.74L
Explanation:
PV=nRT
V=nRT/P
= 0.25mol*0.0821LatmK^-1*273k/1.50atm
=3.74L
Which statement does NOT correctly compare silicon with another element?
Answers
Answer:
i dont know
Explanation:
Answer:
Silicon conducts electricity as well as copper does.
Explanation:
you didn't gave any statements to choose the answer from.
but otherwise, what all I my knowledge says that Silicon conducts electricity as well as copper does.
Grey-coloured iron powder was heated in reddish-brown bromine vapour. A yellowish-green powder was formed. State if the yellowish-green powder is an element, a compound or a mixture. Explain your answer.
Answers
The yellowish-green powder is a compound because two elements (iron and bromine) chemically combined to form it.
What is a compound?A compound in chemistry is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
This means that a compound is formed by the chemical bonding of two or more elements.
According to this question, a grey-coloured iron powder was heated in reddish-brown bromine vapour to form a yellowish-green powder.
The grey-coloured iron is an element that combined with the reddish-brown bromine gas. This suggests that two elements combined. It can be said to be a chemical reaction because a colour change was observed.
Therefore, the yellowish-green powder is a compound because two elements (iron and bromine) chemically combined to form it.
Learn more about compounds at: https://brainly.com/question/13516179
#SPJ1
Can you think of something you do or a hobby you have that is physics
related? Explain your thinking as to how what you do is Physics related.
Answers
You can take physics as a hobby and also it is great that you like both maths and physics as well and also want to be an computer science engineer because physics and math
Chlorine displaces iodine from a solution of sodium iodide in a redox reaction.
The equation for this reaction is shown.
Cl₂ + 2NaI—>2NaCl + I₂
Which statement about this reaction is correct?
A Chlorine is the oxidising agent and it oxidises iodide ions.
B Chlorine is the oxidising agent and it reduces iodide ions.
C Chlorine is the reducing agent and it oxidises iodide ions.
D Chlorine is the reducing agent and it reduces iodide ions.
Answers
Answer:
(A) chlorine is an oxidizing agent in this reaction so it oxidize iodine and it itself is reduced
Explanation:
Cl2 oxi no. = 0 became Cl- oxi no. = -1
so it is reduced
I- oxi no. = -1 became I2 oxi no. = 0
so it oxidized
How many isomers are there in C7H16 ?
a. 6
b. 7
c. 8
d. 9
Answers
Blood is made up of red and white blood cells, platelets and ______.
Find the interquartile range
Answers
blood is made up of red and white blood cells, platelets and plasma
when potassium hydroxide and hydrobromic acid are combined the products are:___
Answers
The reaction between potassium hydroxide and hydrobromic acid results in the formation of potassium bromide and water, with the potassium and bromide ions switching partners.
When potassium hydroxide (KOH) and hydrobromic acid (HBr) are combined, they undergo a neutralization reaction to form potassium bromide (KBr) and water (H2O). The reaction can be represented by the chemical equation:
KOH + HBr → KBr + H2O
In this reaction, the potassium cation (K+) from KOH combines with the bromide anion (Br-) from HBr to form potassium bromide. Meanwhile, the hydroxide ion (OH-) from KOH combines with the hydrogen ion (H+) from HBr to form water.
Potassium bromide is a white crystalline solid that is soluble in water. It is an ionic compound composed of potassium cations and bromide anions. Water is a covalent compound and is formed as a byproduct of the neutralization reaction.
Know more about neutralization reaction here:
https://brainly.com/question/27745033
#SPJ11
The diagram above represents the melting of H2O(s). A 2.00mole sample of H2O(s) at 0°C melted, producing H2O(l) at 0°C. Based on the diagram, which of the following best describes the amount of heat required for this process and the changes that took place at the molecular level?
Answers
Heat : 12 kJ, to maintain hydrogen bonds
Further explanationGiven
A 2.00mole sample ⇒ n = 2 mol
H₂O(s) at 0°C melted, producing H₂O(l) at 0°C
Required
the amount of heat required
the changes that took place
Analysis
Conversion of mol to mass
Use formula of Heat :
Q = mLf (melting/freezing)
Lf=latent heat of fusion (for water=334 J/g)
Solution
mass H₂O(MW=18 g/mol) :
\(\tt mass=2\times 18=36~g\)
Heat required :
\(\tt Q=36\times 334=12024~J\approx 12~kJ\)
The absorbed heat is used to maintain hydrogen bonds in water molecules (there are two hydrogen bonds per molecule)
Paraphrase
The amount of heat required : 12 kJ, and to maintain hydrogen bonds
Count the total number of atoms in the chemical formula 5H2O2
Answers
Answer:
20 atoms
Explanation:
There are 4 in H2O2 because of 2 hydrogens and 2 oxygens.
Then, multiply by 5 because the coefficient is 5, therefore there are 5 H2O2 molecules.
5 x 4 = 20
The total number of atoms in the chemical formula 5H₂O₂ is 20.
What do you mean by the chemical formula ?The term chemical formula is defined as a way of presenting information about the chemical proportions of atoms that represent a particular chemical compound or molecule, applying chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas.
There are three types of chemical formula that are structural, molecular and empirical chemical formula.
There are four atoms in H₂O₂ because of two atoms of hydrogen and two atoms of oxygen. Then, multiply by five because the coefficient is five, therefore there are 5 H₂O₂ molecules.We get,
= 5 x 4
= 20
Thus, The total number of atoms in the chemical formula 5H₂O₂ is 20.
To learn more about the chemical formula, follow the link;
https://brainly.com/question/29031056
#SPJ6
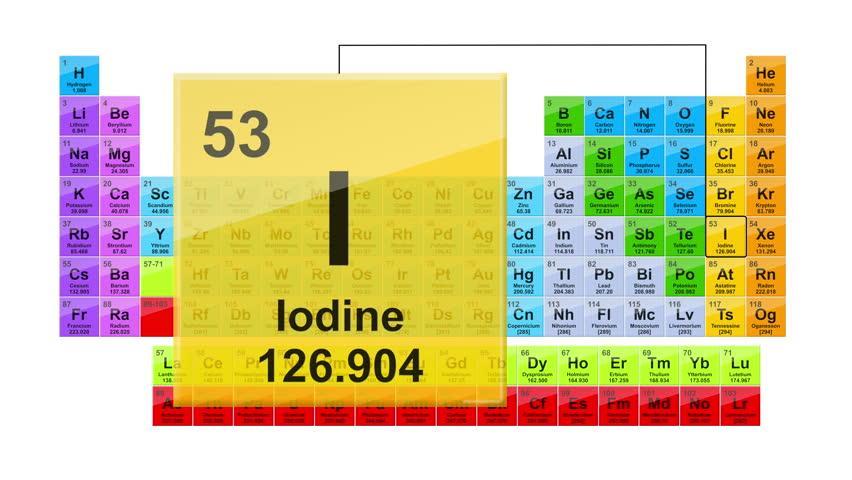
Which element is in Group 17 and has more than 50 protons but less than 75 protons?
Answers
Answer:
Iodine
Explanation:
Iodine is in group 17 on the periodic table (first picture attached). The second picture shows you in the top left-hand corner of Iodine's little square, there is the number 53. This is the atomic number, it is also the number of protons and electrons in an element.
Hope this helped :)

What forces typically hold ions together?
O A. Intermolecular forces
OB. Ionic attractions
OC. Metallic bonds
O D. Covalent bonds
Answers
Answer: Ionic attractions
Explanation:
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
what causes an iron nail to become magnetic when it is rubbed against a permanent magnet over and over again in the same direction
Answers
Answer:
hey! i hope you'll find this helpful, can i have brainliest please? thank you!
When an iron nail is repeatedly rubbed against a permanent magnet in the same direction, it can become temporarily magnetized. This phenomenon is known as magnetic induction.
Iron is a ferromagnetic material, which means it has the ability to be easily magnetized. When the nail is rubbed against the magnet, the magnetic domains within the iron align in a particular direction due to the influence of the magnetic field produced by the magnet. The repeated rubbing in the same direction helps align the magnetic domains more consistently.
The magnetic domains are small regions within the iron where groups of atoms have their magnetic moments aligned. In an unmagnetized iron nail, these domains are randomly oriented, resulting in a net magnetic field of zero. However, when the iron nail is rubbed with a magnet, the magnetic domains align in a common direction, creating a temporary magnetic field within the nail.
The alignment of the magnetic domains persists even after the rubbing stops, causing the iron nail to exhibit magnetism. However, this magnetism is relatively weak and temporary, as the domains can easily revert to their original random orientation. It means that the iron nail loses its magnetic properties over time unless it is exposed to a stronger external magnetic field or is made into a permanent magnet through a different process.
In summary, rubbing an iron nail against a permanent magnet in the same direction aligns the magnetic domains within the iron, resulting in temporary magnetization.
:D
How much water would you need to add to 950 mL of a 3.500 M sodium chloride solution to make a 2.500 M solution?
Answers
You would need to add 250 mL of water.
the empirical formula of a compound is ch2. which could not be the molecular formula? group of answer choices c7h14 c4h8 c12h22
Answers
The empirical formula of a compound is the simplest whole-number ratio of atoms in a molecule of the compound. the possible empirical formula for ch2 among given choices is c. C12H22.
The molecular formula, on the other hand, gives the actual number of atoms of each element in a molecule of the compound. The molecular formula can be a multiple of the empirical formula.
In this case, the empirical formula of the compound is CH2, which means it contains one carbon atom and two hydrogen atoms.
Therefore, the molecular formula of the compound could be C2H4, C4H8, or any other multiple of CH2, but it cannot be C7H14 or C12H22 because these molecular formulas are not multiples of CH2.
So, the correct answer is: c. C12H22.
Learn more about empirical formula here:
https://brainly.com/question/21280037
#SPJ4
Question 70 Marks: 1 Loam is a mixture of gravel, sand, silt, and clay containing what?Choose one answer. a. highly toxic metals b. potassium and ammonium c. decayed plant and animal matter d. dirt
Answers
In addition to these physical characteristics, loam also contains decayed plant and animal matter
Loam is a type of soil that contains a mixture of gravel, sand, silt, and clay. It is considered to be one of the best types of soil for growing plants because of its ability to retain water and nutrients while still allowing for adequate drainage.
which provides organic matter and nutrients that are essential for plant growth. Unlike other types of soil, loam does not contain highly toxic metals that can be harmful to plants and the environment.
Instead, it contains essential minerals such as potassium and ammonium that are important for plant growth. In summary, loam is a healthy mixture of physical and organic components that make it an ideal soil for gardening and farming.
To learn more about : loam
https://brainly.com/question/19413011
#SPJ11
if 1 mol of a fatty acyl-coa containing 20 carbon atoms undergoes three rounds of β oxidation, how many mol of atp are produced from the complete aerobic catabolism of the products of these three rounds? (the remaining acyl-coa is not catabolized further.)
Answers
From the complete aerobic catabolism of the products of three rounds of β-oxidation of 1 mol of a fatty acyl-CoA containing 20 carbon atoms, approximately 60 mol of ATP would be produced.
The complete aerobic catabolism of the products of three rounds of β-oxidation of 1 mol of a fatty acyl-CoA containing 20 carbon atoms can yield a certain amount of ATP. Let's break down the process step-by-step to determine the amount of ATP produced.
1. Each round of β-oxidation involves four key steps: oxidation, hydration, oxidation, and thiolysis. In each round, the fatty acyl-CoA molecule loses two carbon atoms as acetyl-CoA, generating one molecule of NADH and one molecule of FADH2. Therefore, after three rounds of β-oxidation, the fatty acyl-CoA molecule would yield six molecules of acetyl-CoA, three molecules of NADH, and three molecules of FADH2.
2. Each molecule of acetyl-CoA can enter the citric acid cycle (also known as the Krebs cycle or TCA cycle), where it undergoes a series of reactions that generate three molecules of NADH, one molecule of FADH2, and one molecule of GTP (which can be converted to ATP). Therefore, the six molecules of acetyl-CoA produced from the three rounds of β-oxidation would yield 18 molecules of NADH, 6 molecules of FADH2, and 6 molecules of GTP.
3. Each molecule of NADH can generate approximately 2.5 molecules of ATP when it enters the electron transport chain (ETC) and undergoes oxidative phosphorylation. Similarly, each molecule of FADH2 can generate approximately 1.5 molecules of ATP. Therefore, the 18 molecules of NADH would yield approximately 45 molecules of ATP, and the 6 molecules of FADH2 would yield approximately 9 molecules of ATP.
4. The 6 molecules of GTP can be converted to ATP directly, resulting in 6 molecules of ATP.
5. Finally, summing up the ATP generated from NADH, FADH2, and GTP, we have approximately 45 + 9 + 6 = 60 molecules of ATP.
Learn more about aerobic catabolism ere:-
https://brainly.com/question/32271598
#SPJ11
Chemical and physical properties of the seaborgium element
Answers
Answer:
Seaborgium is an artificially produced radioactive chemical element, it's appearance is unknown, it probably has a silvery white or metallic gray colour. The most stable isotope Sg 271 has an half life of 2.4 minutes.