A Downs cell operating at 77.0 A produces 31.0 kg of Na.(b) How many coulombs were passed through the cell?
Answers
1.30×10^8 C were passed through the cell.
Charged material experiences a force when it is exposed to an electromagnetic field due to the physical property of electric charge. You can have a positive or negative electric charge. Unlike charges attract one another while like charges repel one another.
2NaCl ------> 2Na + Cl2
The half reactions show that 2 mol of electrons are gained for every mol of Cl2 gas.
Count the passed coulombs of charge as shown below:
Charge = 673.9 mol Cl2 x (2 mol e 9.65/1 mol of cl2) x (9.65x 10^4/ 1 mol e)
=1.30×10^8 C
The charge passed is therefore 1.30×10^8 C
Learn more about Charge here:
https://brainly.com/question/13871705
#SPJ4
Related Questions
among the trace minerals, which mineral is found in highest concentrations in the body? among the trace minerals, which mineral is found in highest concentrations in the body? zinc copper fluoride iron
Answers
The correct answer is option D.
Among the trace minerals, iron is the mineral that is found in highest concentrations in the body.
In human body, there are 21 trace minerals that are expected to be present including copper, zinc, iron cobalt, manganese, and fluoride.
The function of each trace mineral varies and they play huge role in the growth and development process.
For proper functioning of human body, only small quantities of these minerals are required. Among 21 minerals, iron is found in huge quantities.
Certain foods are rich sources of these minerals. However, deficiency or excess of these minerals can lead to serious health issues.
If you need to learn more about trace minerals click here:
https://brainly.com/question/13023329
#SPJ4
c) Describe a method to determine the mass of dissolved solids in a 100 cm³ sample of
river water.
Page 1 of 2
(4) (Total 7 marks)
3
Answers
A gravimetric analysis is one way to find out how much-dissolved solids there are in a 100 cm3 sample of river water.
What is gravimetric analysis?Generally, One method to determine the mass of dissolved solids in a 100 cm³ sample of river water is through the use of a gravimetric analysis.
This involves first filtering the water sample to remove any suspended solids, then evaporating the remaining liquid to leave behind the dissolved solids.
The remaining solid material can then be weighed, and the mass of the dissolved solids in the original sample can be calculated.
Additionally, chemical analysis can be used to determine the concentration of dissolved solids by using volumetric analysis, by using ion-selective electrodes or by using spectrophotometry.
Read more about gravimetric analysis
https://brainly.com/question/6178384
#SPJ1
6th grade science i mark as brainliest

Answers
Answer:
60 miles
Explanation:
If the car is moving 30 mph, every hour the car has traveled 30 miles. Therefore, if the car travels for two hours, the car has traveled 60 miles if the same speed of 30 mph remained consistent.
Rate of respiration ___ when pyruvate is added to NaF because pyruvate overcomes the inhibition of NaF by increasing the concentration of substrate which is then used in the krebs cycle
Answers
Sodium fluoride (NaF) is a competitive inhibitor of the enzyme enolase, which is involved in the glycolysis pathway of cellular respiration. When NaF is added to a respiration reaction, it can inhibit the production of pyruvate and consequently decrease the rate of respiration.
However, the addition of pyruvate to a NaF-inhibited respiration reaction can overcome this inhibition by increasing the concentration of substrate available for the Krebs cycle. The Krebs cycle, also known as the citric acid cycle, is responsible for producing ATP, NADH, and FADH2 from acetyl-CoA, which is derived from pyruvate.
By increasing the concentration of pyruvate, the rate of respiration can increase because more pyruvate can be converted to acetyl-CoA, providing more substrate for the Krebs cycle. This leads to an increase in the production of ATP and other products of cellular respiration.
Overall, the addition of pyruvate to a NaF-inhibited respiration reaction can overcome the inhibition of NaF by increasing the concentration of substrate available for the Krebs cycle and ultimately leading to an increase in the rate of respiration.
For more such questions on glycolysis
https://brainly.com/question/13948095
#SPJ11
The graph represents the reaction 3H₂ + N₂2NH3 as it reaches
equilibrium. Based on the graph, which two statements about this reaction
are true?
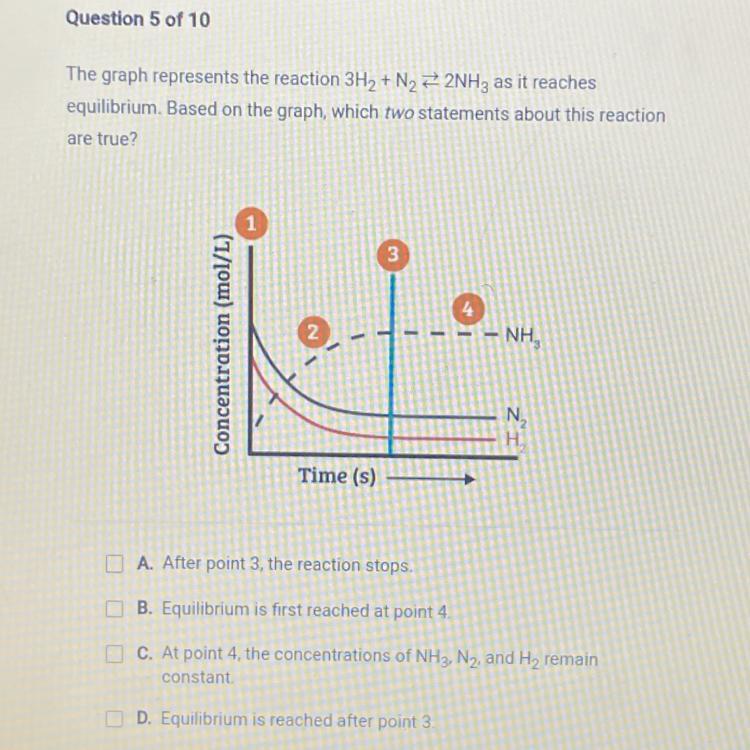
Answers
The statements that are true about the equilibrium position are the statements C and D.
What is equilibrium position?The point at which a chemical reaction has achieved dynamic equilibrium is referred to as the equilibrium position. It is the particular ratio of reactant and product concentrations at which the forward and reverse processes proceed at the same rates, with no overall change in the concentration of the system over time.
In a chemical reaction, reactants are changed into products, and the reaction continues up to the point at which the rates of the forward and reverse reactions are equal.
Learn more about equilibrium:https://brainly.com/question/30807709?
#SPJ1
For the simple reaction of A + B ---> C, if A is the limiting reactant for a certain set of conditions, then B is an excess reactant. True or False
Answers
Answer: True
Explanation:
a 100-g sample of an isotope of sodium, 24na, decays to 30 g after 26 hours. (a) find the half-life of the isotope. (round your answer to two decimal places.) 64.49 incorrect: your answer is incorrect. hours (b) how much of the sample remains after seven hours? (round your answer to two decimal places.) g (c) how long will it take for only 5 g of the sample to remain? (round your answer to two decimal places.) hours
Answers
The half-life of the isotope is 12.8 hours, the amount of the sample that remains after seven hours is 53.47 g, and it will take 92.1 hours for only 5 g of the sample to remain.
The half-life of the isotope can be calculated using the following formula;
\(t_{1/2}\) = (ln 2)/λ
where \(t_{1/2}\) is the half-life, ln 2 is the natural logarithm of 2, and λ is the decay constant. The decay constant will be calculated using the following formula;
λ = (ln(N0/Nt))/t
where N0 is the initial number of nuclei, \(N_{t}\) is the number of nuclei after time t, and t is the time elapsed.
Using the given values, we can write;
N0 = 100 g/(23 g/mol) = 4.348 moles
\(N_{t}\) = 30 g/(23 g/mol) = 1.304 moles
t = 26 hours
λ = (ln(N0/Nt))/t
λ = (ln(4.348/1.304))/26 hours
λ = 0.0542 hours^-1
\(t_{1/2}\) = (ln 2)/λ
\(t_{1/2}\) = (ln 2)/(0.0542 hours^-1)
\(t_{1/2}\) = 12.8 hours
Therefore, the half-life of the isotope is 12.8 hours.
To find out how much of the sample remains after seven hours, we can use the following formula;
\(N_{t}\) = N0 \(e^{(-λt)}\)
where \(N_{t}\) is the number of nuclei after time t, N0 is the initial number of nuclei, λ is the decay constant, and t is the time elapsed.
Using the given values, we can write;
N0 = 100 g/(23 g/mol) = 4.348 moles
t = 7 hours
λ = 0.0542 hours⁻¹
\(N_{t}\) = N0 \(e^{(-λt)}\)
\(N_{t}\) = 4.348 moles \(e^{(-0.0542 hours-1X7 hours)}\)
\(N_{t}\) = 2.327 moles
The mass of the remaining sample can be calculated as:
m = \(N_{t}\) x molar mass of 23Na
m = 2.327 moles x 23 g/mol
m = 53.47 g
Therefore, the amount of the sample that remains after seven hours is 53.47 g.
To find out how long it will take for only 5 g of the sample to remain, we can use the following formula:
\(N_{t}\)= N0 \(e^{(-λt)}\)
where \(N_{t}\) is the number of nuclei after time t, N0 is the initial number of nuclei, λ is the decay constant, and t is the time elapsed.
Using the given values, we can write;
N0 = 100 g/(23 g/mol) = 4.348 moles
\(N_{t}\) = 5 g/(23 g/mol) = 0.217 moles
λ = 0.0542 hours⁻¹
\(N_{t}\) = N0 \(e^{(-λt)}\)
0.217 moles = 4.348 moles\(e^{(-0.0542 hours-1 Xt)}\)
ln(0.217/4.348) = -0.0542 hours⁻¹ x t
t = (ln(4.348/0.217))/0.0542 hours⁻¹
t = 92.1 hours
Therefore, it will take 92.1 hours for only 5 g of the sample to remain.
To know more about half-life here
https://brainly.com/question/24710827
#SPJ4
at standard temperature, the nernst equation can be rewritten to show that the nonstandard cell potential is equal to the standard cell potential minus:select the correct answer below:
a. (0.0257 vn)logq
b. (0.0592 vn)logq
c. (0.0592 vn)lnq
d. none of the above
Answers
The correct answer is b. (0.0592 vn)logq. The Nernst equation relates the cell potential (Ecell) to the concentrations of the reactants and products in the cell.
The correct answer is b. (0.0592 vn)logq. The Nernst equation relates the cell potential (Ecell) to the concentrations of the reactants and products in the cell. At standard conditions (25°C, 1 atm pressure, 1 M concentration), the cell potential is equal to the standard cell potential (E°cell). However, under nonstandard conditions, the Nernst equation must be used to calculate the cell potential. The equation is Ecell = E°cell - (RT/nF)lnQ, where R is the gas constant, T is temperature, n is the number of electrons transferred in the reaction, F is Faraday's constant, and Q is the reaction quotient. At standard temperature (25°C), the equation can be simplified to Ecell = E°cell - (0.0592/n)logQ. Therefore, the nonstandard cell potential is equal to the standard cell potential minus (0.0592/n)logQ.
To know more about Nernst equation visit: https://brainly.com/question/31593791
#SPJ11
can you prepare 100 ml solution of 0.64 m sucrose and 0.58 m nacl using 1 m stock solutions? if yes, write the volume needed of each stock solution. if no, what would you change to prepare this solution?
Answers
Yes, you can use a 1 M stock solution to prepare a 100 ml solution of 0.64 M sucrose and 0.58 M NaCl.
To prepare 100 ml of 0.64 M sucrose, you need 64 g of sucrose (1 M = 1 g/mL). To dissolve 64 g of sucrose in 100 ml of water, you need 64/0.64 = 100 mL of the 1 M sucrose solution.To prepare 100 ml of 0.58 M NaCl, you need 58 g of NaCl (1 M = 1 g/mL). To dissolve 58 g of NaCl in 100 ml of water, you need 58/0.58 = 100 mL of the 1 M NaCl solution.So, you need 100 mL of 1 M sucrose solution and 100 mL of 1 M NaCl solution to prepare 100 ml of 0.64 M sucrose and 0.58 M NaCl solution.
What is the rationale behind the calculation?The rationale behind the calculation is based on the concept of molarity. Molarity refers to the number of moles of solute in one liter of solution. To prepare a solution of a desired molarity, you need to calculate the amount of solute required to reach the desired molarity, and then dissolve that amount of solute in the desired volume of solvent to make the solution.
In this case, you want to prepare 100 ml of a 0.64 M sucrose solution and a 0.58 M NaCl solution. To do this, you need to know how many moles of sucrose and NaCl are required to make 100 ml of these solutions. This is calculated by multiplying the desired molarity (0.64 M for sucrose and 0.58 M for NaCl) by the volume of the solution (100 ml) and converting moles to grams using the molar mass of sucrose (342.3 g/mol) and NaCl (58.44 g/mol).
Once you know the required amount of sucrose and NaCl, you can use 1 M stock solutions to prepare the desired solution. The volume of the 1 M stock solution needed for each solute is calculated by dividing the required amount of solute by the molarity of the stock solution. In this case, since the stock solutions are both 1 M, the required volume of each stock solution is equal to the desired volume of the final solution (100 ml).
Learn more about molarity here: brainly.com/question/17138838
#SPJ4
What is the meaning of a 'DeltaH' value of -400 kJ in a reaction?
OA.
The reaction requires 400 kJ of heat.
OB.
The reaction releases 400 kJ of heat.
OC. The products have 400 kJ of heat energy more than the reactants.
O D. The reaction will not occur.
Answers
Answer: B. The reaction releases 400 kJ of heat.
what is a holiday season to be extra careful about ?

Answers
A woman is swimming across a cold lake. Her body temperature is 98 degrees and the lake water is 60 degrees. Which has more thermal energy, the woman or the lake? Explain your answer!
Answers
I think the lake because of how heat is transferred or something. Just check out the first law of thermodynamics.
Answer:
the woman
Explanation:
Explain why chemical reactions are used to form synthetic materials.
Answers
The rearrangement of the atoms is the reason why.
The synthetic materials form as a product due to chemical reactions with bonds breaking in the process; As the bonds break, the rearrange into these materials.
You're welcome :)
The n = 5 to n = 3 transition in the bohr hydrogen atom corresponds to the __________ of a photon with a wavelength of __________ nm.
Answers
The n = 5 to n = 3 transition in the bohr hydrogen atom corresponds to the emission of a photon with a wavelength of 1280 nm.
Why is Bohr's model limited to hydrogen?Because it does not account for forces owing to inter-electronic attractions, Bohr's atomic model is only valid for single electron species.
The Bohr Model of the Hydrogen Atom presented the planetary model initially, but an assumption about electrons was subsequently established. The premise was that the structure of atoms could be quantized. Bohr hypothesised that electrons orbited the nucleus in fixed-radius orbits or shells.
Electrons orbit the nucleus in fixed-size, fixed-energy orbits. The orbit's energy is proportional to its size. The smallest orbit has the lowest energy. When an electron travels from one orbit to another, it absorbs or emits radiation.
learn more about Bohr Model refer
https://brainly.com/question/18002213
#SPJ4
*
In the table, which is NOT a physical change in size or shape?
(17 Points)
O A.W
B. X
Ос. Ү
O D.Z
Answers
Answer:
Oc. Y is your correct answer
Answer:
k Nishant
Explanation:
the answer is oC . Y
if 4.50 kj of heat is supplied to a 0.560 mol sample of solid copper at 25.0°c, what will the copper’s final temperature be in °c? the specific heat of solid copper is 0.385 j/g • k.
Answers
The final temperature of copper will be approximately 28.92°C.
To find the final temperature of copper, we can use the equation:
q = m × C × ΔT
Where:
q = heat absorbed or released
m = mass of the sample
C = specific heat capacity of the substance
ΔT = change in temperature
First, let's calculate the heat absorbed by the copper using the given information:
q = 4.50 kJ = 4.50 × 10^3 J (converting kilojoules to joules)
m = 0.560 mol × molar mass of copper (Cu) = 0.560 mol × 63.55 g/mol = 35.648 g (converting moles to grams)
C = 0.385 J/g·K
Now we can rearrange the equation to solve for ΔT:
ΔT = q / (m × C)
ΔT = (4.50 × 10^3 J) / (35.648 g × 0.385 J/g·K)
ΔT ≈ 3.92 K
Finally, we can calculate the final temperature by adding the change in temperature to the initial temperature:
Final temperature = 25.0°C + 3.92 K
Final temperature ≈ 28.92°C
To know more about temperature refer here
https://brainly.com/question/46870197#
#SPJ1
PLEASEEE HELP SCIENCE A cold front forms when cold, dense air moves under warm, less dense air, as shown in the
following illustration. Cooler weather usually follows a cold front. Cold fronts often bring other weather
changes.

Answers
An orange contains approximately 10^29 charged particles. why does two oranges repel each other when they are brought together
Answers
Electromagnetic forces are forces that arise between electrically charged particles. They are the result of the interaction between their electromagnetic fields and occur due to the exchange of photons, which carry the electromagnetic force.
These forces can act over long distances and are responsible for various phenomena, including the repulsion or attraction of charged objects.
When it comes to the phenomenon of two oranges repelling each other, it can be explained by the presence of charged particles within the oranges. Oranges contain a large number of charged particles, approximately 10^29. These charged particles consist of negatively charged electrons and positively charged protons.
When two oranges are brought close together, the like charges within them (negative charges from electrons or positive charges from protons) create electromagnetic forces. According to Coulomb's law, like charges repel each other. As a result, the oranges experience a repulsive force that causes them to push away from each other.
In conclusion, the repulsion of two oranges when brought together is due to the electromagnetic forces generated by the like charges within the oranges. The negatively charged electrons and positively charged protons within the oranges create forces of repulsion, causing the oranges to repel each other. This phenomenon can be explained by the principles of electromagnetism and Coulomb's law.
To Learn more about Electromagnetic forces. Click this!
brainly.com/question/14493059
#SPJ11
A pH strip was used to test the pH of a glass of water. The image shows the results.
Use the scale below to determine the pH value of the water, and determine whether the water is acidic, alkaline, or
neutral. Then predict what will happen to the pH if someone were to place a straw into the water and blow.

Answers
The pH strip is used to test the pH of a solution. The pH of water is neutral which is around 7.
What is pH?The pH is known as the power of hydrogen. The pH is used to measure the degree of basicity and acidity of a solution. The amount of hydrogen ion concentration in a solution determines the pH of the solution. Mathematically, pH is given by the formula:
pH -= -log [H⁺]
The pH strip is a strip of litmus paper with which a person can measure the pH value of a liquid solution. The substance in the pH paper causes the paper to show a different color at different acidity values. The official pH scale is between the pH values of 0 to 14, where 0 is very acidic and 14 very alkaline and 7 is neutral pH.
Learn more about pH here:
https://brainly.com/question/15289714
#SPJ1
Answer:
The pH value of the water is 7. And I don't exactly know what would happen if you put a straw into it and blew into it, but if I had to make a guess then I would guess that the pH value would go down because the water is moving around.
How many moles are in 10 g aspartame?
Answers
A substance has 0. 0152088 moles per 10 grammes. One can use the equation grams = weight / molar mass to determine this. The volume of one mole of a chemical is represented by its molar mass on the chemical elements.
You would take its weight (10g) and multiply it by the material's molar mass to determine the number of molecules within 10g of a substance. For instance, if the material is water, its molar mass is 18. 015 g/mol, meaning that 10 g is comparable to 0. 555 molecules of water.
However, the formula moles = mass / molar weight can be used to determine the number of moles in an unit weight regardless of the substance. Therefore, there are 0. 0152088 mole in 10
To know more about Weight click here
brainly.com/question/10069252
#SPJ4
The percent of P in Li3PO4 is
Answers
For example, if we take the atomic masses of Li and P to be 6.9 and 31, respectively:
Mass of P = 31 g/mol
Mass of Li3PO4 = 3 * 6.9 + 31 = 121 g/mol
Percentage of P = (31 / 121) * 100 = 25.62 %
So, the percent of P in Li3PO4 is approximately 25.62%.
*They allow atoms to____ and form____or_____ substances. The number of valence electrons determines how an element reacts with other elements. For the first 3 rows: To find the number of valence electrons for elements in the first three rows, count how far along in a row the element is, and that number is the number of valence electrons. Many _______depend on valence electrons.
Answers
The number of valence electrons for neutral atoms is the same as the number of the atom's main group. A periodic table element's column can be used to determine its main group number. For instance, carbon, which belongs to group 4, has four valence electrons.
For an atom to become a charged ion and create an ionic connection with another atom, it must have between one and three valence electrons, which it will lose.
Atoms can be very reactive or extremely inactive depending on how many valence electrons they have. The quantity of valence electrons in an atom also affects whether or not it is more likely to lose or acquire electrons during chemical processes. Metals may carry electricity because they readily give away electrons.
Learn more about valence electrons https://brainly.com/question/12717954
#SPJ9
helpe pls I will mark brainlest

Answers
Answer:
What is the question needing to be answered?
Explanation:
Usually with a scientific hypothesis, you ask the question in an if, then statement. For example: If it rains outside, then the ground will be wet. It's kind of like a cause and effect statement.
what does this nmr data indicate about the purity of the product pinacolone, a ketone molecule with one keto carbonyl group, four long-chain carbons, and two branched ? use three key signals to justify your answer.
Answers
Using the three key signals, it indicates that the pinacolone product is pure, if all three key signals are observed without any unexpected peaks.
Based on the provided NMR data for pinacolone, we can analyze the purity of the product using three key signals. Pinacolone is a ketone molecule with one keto carbonyl group, four long-chain carbons, and two branched carbons.
1. The first key signal to look for is the presence of a carbonyl group (C=O) in the chemical shift range of 200-220 ppm. A sharp peak in this region indicates the keto carbonyl group is present, which is a characteristic feature of pinacolone.
2. The second key signal corresponds to the four long-chain carbons, which typically appear in the 20-40 ppm range. Peaks in this region suggest the presence of these carbon atoms, contributing to the molecular structure of pinacolone.
3. The third key signal is related to the two branched carbons. These carbons usually show up in the 10-30 ppm range in the NMR spectrum. Peaks within this region indicate that the branched carbons are present in the pinacolone molecule.
If all three key signals are observed without any unexpected peaks, it indicates that the pinacolone product is pure. Conversely, the presence of extra peaks in the NMR spectrum may suggest impurities or side products in the sample.
More on pinacolone: https://brainly.com/question/30888003
#SPJ11
True or False:
Assuming argon behaves like an ideal gas, 4.00 g of argon gas was found to occupy a volume of 1.05 L at a pressure of2.80 atm. Therefore, the temperature of the gas is 84.9 ℃.
Answers
The given statement is false. The problem can be solved using the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in kelvin. Rearranging this equation gives T = PV/nR.
In the given problem according to ideal gas law, we have P = 2.80 atm, V = 1.05 L, and n = 4.00 g / (39.95 g/mol) = 0.100 mol (using the molar mass of argon). The gas constant R is 0.08206 L·atm/(mol·K). Substituting these values into the equation for T gives:
T = (2.80 atm)(1.05 L)/(0.100 mol)(0.08206 L·atm/(mol·K)) = 334 K
Converting this temperature to degrees Celsius gives:
T = 334 K - 273.15 = 60.9 ℃
Therefore, the statement is false. The temperature of the gas is actually 60.9 ℃, not 84.9 ℃.
To learn more about Ideal gas law click here
https://brainly.com/question/30458409
#SPJ11
Calculate poH if given pH of 9.3
14
4.7
5.01 x 10^-6
Answers
Answer: Option (b) is the correct answer.
Explanation:
It is known that,
pH + pOH = 14
So, pOH = 14 - pH
As the given pH is 9.3. Hence, pOH for the given solution will be calculated as follows.
pOH = 14 - 9.3
= 4.7
Therefore, pOH of the given solution is 4.7.
0.764 km to centimeters
Answers
Answer: 76400cm
Explanation:
How many molecules are in 0.500 mole of N2O5?
Answers
Answer:
3,011.10e23.
Explanation:
Mole measure the number of elementary entities of a given substance that are present in a given sample. 3.01×10²³molecules are in 0.500 mole of N₂O₅.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number. Mole is directly proportional to given mass.
Mathematically,
Number of molecules/atoms/ions of N₂O₅ = number of moles×6.022×10²³(Avogadro number)
Number of moles of N₂O₅= 0.500 mole
Substituting the given values, we get
Number of molecules/atoms/ions =0.500moles×6.022×10²³(Avogadro number)
Number of molecules/atoms/ions of N₂O₅ =3.01×10²³molecules.
Therefore, 3.01×10²³molecules are in 0.500 mole of N₂O₅.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2
An experiment is carried out to determine the molar mass of a compound by the freezing point depression method using the equation mass solute MM7.05 AT x kg solvent The data below are collected Mass of empty test tube Mass of test tube and solvent Mass of solute dissolved in solvent 2.000 g Freezing point of pure solvent Freezing point of solution How many significant figures can be reported for the molar mass of the solute? 42.0 g 73.6 g 78.1 °C 77.6 °C (A) I (B) 2 (C) 3 (D) 4
Answers
The molar mass of the solute is calculated using the equation Mass solute MM7.05 AT x kg solvent.
What is solvent?A solvent is a substance that can dissolve or disperse one or more other substances, usually to form a homogenous mixture. Solvents are commonly used to dissolve substances such as pigments, dyes, inks, and resins, which are then used in a variety of products including paints, coatings, adhesives, and cleaning products. Solvents are also used to dissolve other solvents, such as ethanol in water, to form a more concentrated solution. Solvents are widely used in industry as well as in everyday life, and come in a number of different forms such as liquids, gases, and solids. The most commonly used solvents are organic liquids, such as alcohols and hydrocarbons like benzene and toluene.
Since the mass of the solute is given to two significant figures (42.0 g) and the freezing point of the solution is given to one significant figure (77.6 °C), the molar mass of the solute can be reported to two significant figures.
Therefore, the correct option is B.
To learn more about solvent
https://brainly.com/question/25326161
#SPJ1
Whta is a major difference between the rutherford and the wave mechnaical models of the atom?
Answers
A major difference between the Rutherford atomic model and the Wave mechanical model of atom is that Rutherford model was based on the gold foil experiment which considered only the particle properties of atoms whereas the wave mechanical model considers the particle properties and the wave properties of atoms.
Rutherford atomic model was given by Ernest Rutherford to explain what an atom is. His gold foil experiment showed that atom is mostly empty space with, small, dense, positively-charged nucleus.
Wave mechanical model was given by Thomas Young. He called the experiment as Double-slit experiment, to test the nature of the light. Young discovered that electrons of the atom exhibited the particle-wave dual properties which led to this theory named as wave mechanical theory of an atom.
These theories explain different properties of atoms with the help of different experiments.
To learn more about Rutherford model of atom,
brainly.com/question/2031894
#SPJ4