Rupert had three substances. A brown substance was a liquid at room
temperature. He hit each of the other two with a hammer. A blue crystal
cracked but did not break. A silver substance flattened but did not crack.
Which two statements could be true?
A. The brown substance is ionic
B. The silver substance is ionic
C. The brown substance is molecular
D. The blue substance is ionic
Answers
Answer:
its C and D
C. The brown substance is molecular
D. The blue substance is ionic
Explanation:
did the test !
Two correct statements are B) The silver substance is ionic
C) The brown substance is molecular.
What kind of substance is silver?Silver is a chemical element with the symbol Ag and atomic wide variety 47. categorized as transition steel, Silver is stable at room temperature.
Which substance is molecular?It is a molecular substance, that's a substance with or more atoms, the smallest gadgets of remember joined together via a covalent bond. A covalent bond is a hyperlink created via the sharing of electrons that holds these atoms collectively.
Learn more about substances here: https://brainly.com/question/2901507
#SPJ2
Related Questions
PLEASE HELP, IS MY ANSWER CORRECT?
How does the ground temperature in sunlight with CO2 compare with the ground temperature in sunlight without CO2 (part A)? is my answer correct?
Based on the thermometer provided, it is clearly visible that when the simulation is without CO2, the temperature goes higher, however, not as quickly as when CO2 IS present.
Answers
Based on the thermometer provided, the ground temperature in sunlight with CO2 rises more rapidly and reaches a higher temperature compared to the ground temperature in sunlight without CO2.
Which biomolcule is important for the make up of RNA and DNA?
A. Nucleic Acids
B. Proteins
C. Carbohydrates
D. Lipids
Answers
Answer:
A. Nucleic Acids
Explanation:
This is because the nucleotides combine with each other to form a polynucleotide know as DNA or RNA.
PLZ HELP ASAP
Which would increase the reaction rate?
Check all that apply.
A. Stirring the reaction
B. Raising the activation energy
O C. Adding a catalyst
D. Raising the temperature
Answers
Answer:
adding a catalyst will increase the reaction rate.
Explanation:
im like 95% sure thats right.
Answer:
A. C. D
Explanation:
ap ex
A sample of a gas at 25°C has a volume of 150 mL when its pressure is 0.947 atm. What will the temperature of the gas be at a pressure of 0.987 atm and changes to 144mL?
*please help*
Answers
Answer:
25°C
Explanation:
Combined Gas Law (P₁V₁)/T₁ = (P₂V₂)/T₂
(0.947 atm)(150 mL)/25°C = (0.987 atm)(144mL)/T₂
5.682 = 142.128/T₂
T₂ = 142.128/5.682
T₂ = 25.0137272756°C = 25°C
A student analyzed a sample of sea water and found that it contained 2.3g of NaCi, 0.005g of MgSo4, 0.234g of CaCI2 and 60.12g of H2O. Total mass of the sample is
Answers
\(2.3+0.005+0.234+60.12=\boxed{62.659 \text{ g}}\)
Which of the following statements is true regarding soundwaves?
a. Soundwaves travel as longitudinal or transverse waves depending on the medium they’re traveling through.
b. Soundwaves travel as transverse waves only.
c. Soundwaves travel as longitudinal or transverse waves depending on the temperature of the medium.
d. Soundwaves travel as longitudinal waves only.
Answers
Answer: Soundwaves travel as longitudinal or transverse waves depending on the temperature of the medium.
Explanation: i think its C
Will ag2so4 precipitate when 100ml of .050M agno3 is mixed with 10ml of 5x10-2m na2so4 solution
Answers
No, Ag₂SO₄ will not precipitate when 100ml of .050M AgNO3 is mixed with 10ml of 5x10-2 m Na₂SO₄ solution because the precipitate is made only in an aqueous solution.
What is precipitation?Precipitation is the solid extract that is collect in a place. Precipitate is the concentration of the substance in a solution in a specific place.
Thus, No, Ag₂SO₄ will not precipitate when 100ml of .050M AgNO3 is mixed with 10ml of 5x10-2 m Na₂SO₄ solution because the precipitate is made only in an aqueous solution.
Learn more about precipitation
https://brainly.com/question/18109776
#SPJ1
3. Which of the following describes the author's purpose in the text? R1.6
A.
to encourage students not to think about money when they're
choosing a degree or career to pursue
B. to stress the importance of viewing college as a way to develop jobs
skills, as well as determine what is important to you
C. to discourage students from pursuing careers in business, as people
are generally dissatisfied with their lives
D. to encourage students to pursue careers that help other people, as
they are usually the most enjoyable and meaningful
Answers
Answer:
B
Explanation:
I took the assessment
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
A 1.0 L buffer solution is 0.300 M HC2H3O2 and 0.045 M LiC2H3O2. Which of the following actions will destroy the buffer?
a) Adding 0.050 moles of HC2H3O2
b) Adding 0.075 moles of HCl
c) Adding 0.0500 moles of LiC2H3O2
d) Adding 0.050 moles of NaOH
e) None of the above will destroy the buffer.
Answers
Answer:
b) Adding 0.075 moles of HCl
Explanation:
A buffer is defined as the aqueous mixture of a weak acid and its conjugate base or vice versa (Weak base with its conjugate acid).
The buffer of the problem is the acetic acid / lithium acetate.
The addition of any moles of the acid and the conjugate base will not destroy the buffer, just would change the pH of the buffer. Thus, a and c will not destroy the buffer.
The addition of an acid (HCl) or a base (NaOH), produce the following reactions:
HCl + LiC₂H₃O₂ → HC₂H₃O₂ + LiCl
The acid reacts with the conjugate base to produce the weak acid.
And:
NaOH + HC₂H₃O₂ →NaC₂H₃O₂ + H₂O
The base reacts with the weak acid to produce conjugate base.
As the buffer is 1.0L, the moles of the species of the buffer are:
HC₂H₃O₂ = 0.300 moles
LiC₂H₃O₂ = 0.045 moles
The reaction of HCl with LiC₂H₃O₂ consume all LiC₂H₃O₂ -because there are an excess of moles of HCl that react with all LiC₂H₃O₂-
As you will have just HC₂H₃O₂ after the reaction, the addition of b destroy the buffer.
In the other way, 0.0500 moles of NaOH react with the HC₂H₃O₂ but not consuming all HC₂H₃O₂, thus d doesn't destroy the buffer.
Question 4 of 30
Scientific research shows that Earth's climate is changing due to human
activities. How can scientific research on climate change help society?
A. It can help us stop storms before they occur.
B. It can help us find a new way to make more water.
C. It can help us track how quickly elements of the climate are
changing
D. It can help us reverse the effects of climate change.
th
Answers
C. It can help us track how quickly element of the climate are changing
important of science
Answers
Answer:Science is important because science
is the the way to know about other things that we didn't know about our planet and many other things.
Explanation:thank you hope it
Which is an important question to consider when creating an environmental policy?
O How can it reduce the need for green spaces?
How can it help to increase human impact on resources?
O is the benefit worth the cost?
O How will it reduce tourist activity?
Answers
Answer: is the benefit worth the cost?
Explanation: for those on edge :)
Answer:
Is The Benefit Worth The Cost?
Explanation:
What’s a carbohydrate?
A. A hormone
B. A cholesterol molecule
C. An enzyme
D. A sugar molecule
Answers
using the calibratiomn curve below, determine the concentration of copper sulfate solution if its absorbance reading is 0.280 at 650mm.
Answers
The concentration of copper sulfate solution is 11ppm.
The calibration curve for copper sulfate is shown in the figure below.
copper sulfate is a compound of copper and sulfuric acid with the chemical formula CuSO4.To find the concentration of copper sulfate solution with an absorbance reading of 0.280 at 650 mm, we can use the equation:
Concentration (ppm) = m x absorbance + b,
where m is the slope of the graph and b is the y-intercept of the graph.
Using the graph, we can calculate m and b as follows:
\(m =\frac{ (20 - 0)}{(0.750 - 0.250)} = 40\\b = 0 - (40 *0.250) = -10\)
Therefore, the concentration of copper sulfate solution with an absorbance reading of 0.280 at 650 mm is:
Concentration (ppm) = \(40 * 0.280 - 10 = 11 ppm\)
learn more about copper sulfate Refer:brainly.com/question/22560035
#SPJ4

Balance the following equations:
1) C2H402+_02->_C02+ _H20
2) V205+ _CaS→_Ca0+ V2S5
3) S8+_O2->_SO2
Answers
Answer:
1) 0 C2H4O2 + 0 O2 -> 0 CO2 + 0 H2O (balanced)
2) V2O5 + CaS -> CaO + V2S5
just additional info: V2O5 is divanadium pentaoxide
LHS (Left hand side)
V: 2
O: 5
Ca: 1
S: 1 x 5 [to balance with the right hand side of the equation]
RHS (Right hand side)
V: 2
O: 1 x 5 [to balance with the left hand side of the equation]
Ca: 1
S: 5
When you balance any elements, you have to balance the whole chemical compound.
Thus,
V2O5 + 5 CaS -> 5 CaO + V2S5
LHS CHECK:
V: 2
O: 5
Ca: 5
S: 5
RHS CHECK:
V: 2
O: 5
Ca: 5
S: 5
3) S8 + O2 -> SO2
LHS:
S: 8
O: 2
RHS:
S: 1 x 8 [to balance with LHS]
O: 2
When you balance any elements, you have to balance the whole chemical compound.
S8 + O2 -> 8 SO2
When we add 8 to the RHS, it gives us 8S, 16 O.
In order to balance that into the RHS, I need to multiply the O2 by 8, which will give 8(O2) = 16 O particles.
Therefore, S8 + 8 O2 -> 8 SO2 is the final answer for (3).
Use the equation for Charles’s Law to calculate the following. (2pts)
A plastic bottle is filled with a gas to the volume of 0.50 L at a temperature of 294.15k. The bottle then placed in a freezer for 24 hours at a temperature of 273.15k.
What is the volume of gas inside the plastic bottle when it is taken out of the freezer?
Answers
After the plastic bottle is taken out of the freezer, the amount of gas within is 0.465 L.
What occurs to empty plastic bottles that have been in the freezer for a while?The cooling of the air causes the air to contract. Due to the reduced pressure, the air inside the bottle constricts. The bottle falls as a result of external pressure.
When you remove cold water from the refrigerator, what will happen to it?When a cold water bottle is removed from the refrigerator, the surrounding air's water vapour makes contact with the bottle's cold surface and condenses. As a result, water transitions from a gas to a liquid form.
To know more about gas visit:-
https://brainly.com/question/14812509
#SPJ1
What would be the volume in liters of an 25.15 liter sample of gas at 201 °C and 2.31 atm if conditions were changed to STP?
Answers
The volume of the gas at STP would be 23.93 liters.
The volume of gas at STP (Standard Temperature and Pressure), we need to use the Ideal Gas Law, which states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. First, we need to calculate the number of moles of gas in the initial sample. We can use the formula n = PV/RT, where P is the initial pressure, V is the initial volume, R is the gas constant, and T is the initial temperature.
n = (2.31 atm) x (25.15 L) / [(0.0821 L atm/mol K) x (201 + 273.15 K)]
n = 1.067 moles
Now, we can use the molar volume of gas at STP, which is 22.4 L/mol, to calculate the volume of gas at STP.
V = n x 22.4 L/mol
V = 1.067 moles x 22.4 L/mol
V = 23.93 L
Therefore, the volume of the gas at STP would be 23.93 liters.
For more such questions on gas
https://brainly.com/question/25736513
#SPJ11
Which shows an isomer of the molecule below?

Answers
Answer:
The answer is B, in this case.
Explanation:
An isomer is a molecule with the same number of atoms as another compound, but they differ in arrangement of the atoms.
(Please someone help me!) (No links!)
Please ignore the blue dot, I accidentally pressed it and won't get rid of it!
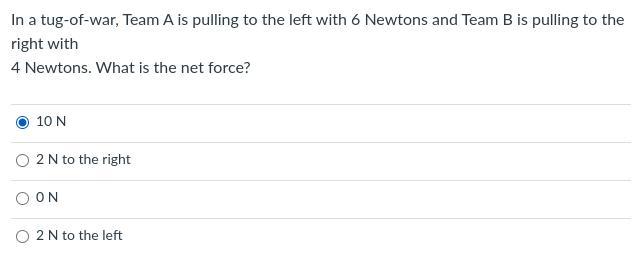
Answers
Answer:
you picked the right one the net worth of force would be 10
Explanation:
how cuz you got 1 team with 6 force then aother 1 with 4 add em up u get 10
Which would occur if a ball x were rolled across a smooth floor to collide with a similar ball y that was stationary
Answers
Answer:
For a collision where objects will be moving in 2 dimensions (e.g. x and y), the momentum will be conserved in each direction independently (as long as there's no external impulse in that direction). In other words, the total momentum in the x direction will be the same before and after the collision.
Explanation:
Q: How does the starting
position affect the speed of a
ball rolling down a ramp?
I NEED HELPP PLSSS ASAP!!!
Answers
The force of gravity points straight down, but a ball rolling down a ramp doesn't go straight down, it follows the ramp. Therefore, only the component of the gravitational force which points along the direction of the ball's motion can accelerate the ball. The other component pushes the ball into the ramp, and the ramp pushes back, so there is no acceleration of the ball into the ramp. If the ramp is horizontal, then the ball does not accelerate, as gravity pushes the ball into the ramp and not along the surface of the ramp. If the ramp is vertical, the ball just drops with acceleration due to gravity. These arguments are changed a bit by the fact that the ball is rolling and not sliding, but that only affects the magnitude of the acceleration but not the fact that it increases with ramp steepness.
Antimony reacts with sulfur to form Sb2S3.
What is the percentage yield for the reaction in which 1.40 g of Sb2S3 is obtained from 1.73 g of antimony and a slight excess of sulfur?
Answers
Answer:
.2sb
Explanation:
according to my mind its the percentage of the question
Question: A first order reaction : B===>C has a half life of 20mins. What percentage shall have reacted after 47minutes
Answers
Answer:19.6%
Explanation:
From K=0.693/t
0.693/20 =0.03465min^-1
But rate law is K =2.303/t log a/(a-x)
Substitute the value of k
0.03465=2.303/47 log a / (a-x)
Log a/ (a-x) = 0.7071
a/ (a-x) = 5.094
(a-x)/ a = 1/5.094 = 0.196
Percentage of reactants remaining after 47 minutes= 0.196× 100% = 19.6%
(Al = 27.0 g, O = 16.0 g, H = 1.0 g)
2 Al(OH)3 Al2O3 + 3 H2O
how many grams are produced from .85 moles of AI(OH)3
Answers
Answer:
Explanation:
21
the electron geometry of a moeluclar who has a central atom with four single bonds and one lone pair of electrons is which of the following
Answers
The molecular geometry of a molecule whose central atom has four single bonds and two lone pairs of electrons is square planar.
The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape. If there are only two bonds and one lone pair of electrons holding the place where a bond would be then the shape becomes bent. For sp3 hybridized central atoms the only possible molecular geometry is tetrahedral. If all the bonds are in place the shape is also tetrahedral.
To learn more about molecular geometry check the link below:
https://brainly.com/question/19866552
#SPJ4
What is the charge of an atom's nucleus if it has 20 protons, 21 neutrons, and 20 electrons?
Answers
Term an atom that contains 20 protons, 21 neutrons and 20 electrons has: Definition "no charge" If an atom is neutral there is one electron for every proton.
In a coffee cup calorimeter, 25.0 g of zinc are added to 100. g of water. The temperature of the water increases from 20.0°C to 27.5°C. What was the initial temperature of the zinc sample?
Answers
The initial temperature is 294.34°C.
The heat that the sample of Zinc gives is equal to the heat that water is absorbing. That is:
C(Zn) * m(Zn) * ΔT(Zn) = C(H2O) * m(H2O) * ΔT(H2O)
Where:
C is specific heat (Zn: 0.390J/g°C; H2O: 4.184J/g°C)
Given:
m is mass (Zn: 25.0g; H2O: 100.0g)
ΔT (Zn: ?; H2O: (27.5°C - 20.0°C = 7.50°C)
Replacing:
0.390J/g°C * 25.0g * ΔT(Zn) = 4.184J/g°C * 100.0g * 7.50
ΔT(Zn) = 321.84°C
As final temperature of Zn is 27.50°C, initial temperature is:
Initial temperature: 321.84°C + 27.50°C = 294.34°C
Hence, the initial temperature of the zinc sample at a specific heat is 294.34°C.
To know more about temperature, refer: https://brainly.com/question/15520591
#SPJ1
The typical dosage of statin drugs for the treatment of high cholesterol is 10 mg. Assuming a total blood volume of 4.5 L, calculate the concentration of drug in the blood in units of % (w/v)
Answers
Answer:
1.904 ppm
Explanation:
Concentration of drug in units of ppm = mass of solute / (mass of solution ) × 1000000
mass of blood = density of blood × volume = 1.05 g / ml × 5000 ml = 5250 g
mass of solution = mass of blood + mass of solute ( statin) = 5250 + 0.01 g = 5250.01 g
Concentration of drug in units of ppm = (0.01 g / 5250.01 g) × 1000000 = 1.904 ppm
I hope this helps!!
A nuclear power plant is scheduled to undergo a retrofitting. What is the most likely outcome of the change?
The power plant will no longer generate radioactive wastes.
The power plant will recycle the water it uses to cool the reactor.
The power plant will be able to reprocess its used uranium fuel.
The power plant will switch from fission reactions to fusion reactions.
Answers
Answer:
It's B
Explanation:
I got it right on edjenuity
The most likely outcome of the retrofitting of a nuclear power plant is that it will be able to reprocess its used uranium fuel. Therefore, option C is correct.
What is nuclear power plant?A nuclear power plant is a facility that generates electricity by harnessing the heat produced by nuclear reactions. At the heart of a nuclear power plant is the reactor, where a controlled chain reaction of nuclear fission occurs.
Reprocessing is a process by which the used nuclear fuel is chemically treated to separate out and recover the unused uranium and plutonium, which can then be reused as fuel. This reduces the amount of radioactive waste produced by the plant and also extends the life of the uranium fuel.
The fission reactions to fusion reactions is not currently a viable option for power generation, and the idea that a retrofitting would eliminate radioactive wastes is unlikely, as nuclear power plants will always produce some amount of radioactive waste.
Thus, option C is correct.
To learn more about the nuclear power plant, follow the link:
https://brainly.com/question/4246037
#SPJ3