Jimmy's Chemical reaction/ Recipe for making Grilled chese sandwiches is given below: 1 slice of cheese + 2 slices of bread = 1 Grilled chese sandwich ( mole ratio is, 1:2:1) If Jimmy has the following amounts of ingredients (reactants/ reagents) : 10 slices of cheese 30 hslices of bread Answer the questions below in your submission: a) What is the limiting reagent/reactant? b) what is the excess reactant? c) True or false? The number of glilled chese sandwishes he can make is decided by the limiting reactant because it gets used up most.
Answers
Answer:
See explanation
Explanation:
We have been told in the question that the equation of the reaction is; 1 slice of cheese + 2 slices of bread = 1 Grilled cheese sandwich ( mole ratio is, 1:2:1) .
Then the reagents are 10 slices of cheese 30 slices of bread. It then follows that 10 slices of cheese should be combined with 20 slices of bread according to the mole ratio.
However, we have 30 slices of bread and 10 slices of cheese so cheese is the limiting reactant while bread is the reactant in excess.
Yes, the number of glilled chese sandwishes he can make is decided by the limiting reactant because it gets used up most.
Related Questions
Define matter and provide some examples of different states of matter
Answers
The three states of matter are solid - example is stone, liquid - example is water and gas - example is air.
What is matter?A matter is referred to as a substance which has a certain mass and takes up a certain volume in space.
For example pen, pencil, toothbrush, water, milk are matters as well as car, bus, bicycle is also a matter. So matter is considered as a living thing and a non-living thing.
There are three states of matter and they include;
solid - example is stoneliquid - example is watergas - example is airThey have different properties, which can be explained by looking at the arrangement of their particles. This is the theoretical temperature at which particles have the least amount of energy and the slowest movement.
Learn more about states of matter here: https://brainly.com/question/9402776
#SPJ1
which compound is an ester? ii , not selected v , not selected correct answer: iii iv , not selected i
Answers
Compound III is the ester in the given options.
To identify the ester among the compounds provided, we need to understand the characteristics of an ester. Esters are organic compounds that are formed by the reaction between an alcohol and an organic acid, resulting in the elimination of water. They have the general structure R-COO-R', where R and R' represent alkyl or aryl groups.
In the given options, compound III, when properly named, is ethyl ethanoate (CH3COOCH2CH3). It consists of an ethyl group (CH3CH2-) attached to the carbonyl carbon of an ethanoate group (-COO-). This structure corresponds to the general structure of an ester.
On the other hand, compounds I, II, IV, and V do not exhibit the characteristics of an ester. Compound I is not selected. Compound II is not an ester, but rather an alkene. Compound IV is not an ester, but rather an amide. Compound V is not an ester, but rather a ketone.
Therefore, compound III (ethyl ethanoate) is the ester among the given options.
Learn more about ester here:
https://brainly.com/question/32098100
#SPJ11
What do the horizontal portions of the graph represent? When heat is added to or removed from the substance and the temperature remains the same, where is the thermal energy going? Why?
Answers
how do I calculate the density

Answers
A) 1.1 g/cm^3
B) .95 g/cm^3
C) 1 g/cm^3
i hope this helps!!!
Using the following diagram, how
would you calculate the Ry for the
lightest material?
A
В.
A. Rf = A/B
B. Rf = BIA
C. Rf = A/C

Answers
Answer: B is the answer
Explanation:I just answered
4 Activity A Chapter 4 Pregnancy and Birth Nutrition and Lifestyle Choices During Pregnancy Name Date Period Sam and Elise have been married for one year. Until now, they have not considered babies or pre- natal development when making lifestyle choices. Sam and Elise recently learned, however, that she is pregnant and is expecting to have twins. This presents many new choices and changes the couple must make. Sam and Elise are both excited and anxiously awaiting the birth of their children. Read each scenario presenting various options for Sam and Elise. Indicate which option may be best and explain your response in the space provided. 1. Sam and Elise are at Sam's family reunion this summer. Sam has a large family, and many of his family members smoke cigarettes. Around lunchtime, the party has split into two groups. The group outside has a pleasant view, but many are smoking. The group sitting indoors is smaller, but no one is smoking Which environment is best for Elise to eat her lunch? Why? 2. Sam and Elise are at a restaurant. Today's daily specials include rare steak, swordfish, and vegetable pasta. Each specialty comes with salad and fruit. Elise favors all three of these dishes. Which meal choice is best for Elise? What health risks are associated with the other two dishes? 3. Now that Elise is pregnant, Sam and Elise are considering moving out of their current home and into a new, larger one. Elise's sister, Amalia, told the couple about a house for sale next door to her that Elise has always admired. Amalia, however, lives hours away from Sam and Elise's friends and other family Sam and Amalia also argue much of the time when they are together, which upsets Elise. If Sam and Elise move next door to Amalia, how might this affect Elise emotionally and physically? 4. In their search for a new home, Sam and Elise find an interesting house built in the early 1920s The house, however, has not had many updates, including the walls. The couple is considering buying the house and then redecorating and remodeling it as a project What health hazards could the house potentially pose to Elise?
Answers
The best environment for Elise to eat her lunch would be indoors with the smaller group where no one is smoking. Smoking and exposure to secondhand smoke can have harmful effects on both the mother and the developing babies. It is important for Elise to avoid exposure to cigarette smoke during pregnancy as it can increase the risk of complications such as low birth weight, premature birth, and respiratory issues for the babies.
Therefore, choosing the smoke-free environment indoors would be the best option for Elise and the twins' well-being.
The best meal choice for Elise would be the vegetable pasta with salad and fruit. During pregnancy, it is recommended to avoid rare or undercooked meats and fish due to the risk of foodborne illnesses, such as salmonella or listeria, which can harm the developing babies. Swordfish is known to have higher levels of mercury, which can be harmful to the babies' nervous system. Therefore, choosing the vegetable pasta, which is a safe and nutritious option, would be the best choice for Elise and the twins.
Moving next door to Amalia, considering their strained relationship and frequent arguments, could have negative emotional and psychological effects on Elise. Pregnancy is a sensitive time, and stress can impact the mother's well-being and potentially affect the babies' development. It is important for Elise to have a supportive and stress-free environment during pregnancy. Living next to Amalia, with the distance from friends and family, and the presence of ongoing arguments, may increase stress levels for Elise, potentially impacting her emotional and physical health.
The house built in the early 1920s with few updates may pose potential health hazards to Elise. One concern could be lead-based paint, which was commonly used in older homes. Ingesting or inhaling lead particles can be harmful to both the mother and the babies, as it can affect the development of the nervous system. Additionally, the house might have other issues such as mold, asbestos, or poor ventilation, which can also have negative health impacts. It is important for Elise and Sam to thoroughly inspect and address any potential health hazards before considering buying and remodeling the house, ensuring a safe and healthy living environment for the pregnancy.
For more such questions on smoking
https://brainly.com/question/29110837
#SPJ8
Write the first five members of Alkanes
Answers
Ethane (C2H6)
Propane (C3H8)
Butane (C4H10)
Pentane (C5H12)
10. How many molecules in this chemical equation?
3(OH)2 A 3, B 6, C 7, D 2
Answers
Answer:
B, 6
Explanation:
How do you separate a mixture of sodium chloride and potassium trioxonitrateV
Answers
Answer:
See explanation
Explanation:
We separate substances based on their important properties such as reaction to heat, solubility in water, magnetic properties, etc.
To separate sodium chloride and potassium trioxonitrateV, we need to heat the solution. KNO3 dissolves at a high temperature and crystallizes out as the solution is cooled.
Hence when we heat the solution, KNO3 dissolves, as we cool the solution, solid KNO3 crystals are obtained while NaCl remains in solution. We have now separated the two salts in the solution.
Note that the solubility of NaCl is almost independent of temperature.
Complete the mechanism for this reaction by drawing the intermediates formed and adding the missing curved arrow notation.
Answers
When hydrochloric acid dissolves in water, the hydronium ion is created. Analyzing the developing bond modifications is helpful. Hydrochloric acid is acting as an acid, and water is acting as a base. Take into account the variations in bonding between the raw materials and the finished goods.
The hydronium ion (H3O+) observed in the products was produced by joining one of the oxygen atom's lone pairs to a hydrogen atom. A chloride ion was created as a result of the hydrogen-chlorine bond in HCl being broken and the electrons in this bond turning into a lone pair on the chlorine atom. The overall charge of the reactants is the same as the overall charge of the products.
This demonstrates how the new H-Cl bond is created by joining an electron-poor hydrogen atom of the hydronium ion to a lone pair of electrons from the electron-rich chloride ion. The oxygen-hydrogen link breaks because hydrogen can only make one bond, leaving the electrons of the oxygen atom with a lone pair.
To know more about the polar reaction mechanism click here:
brainly.com/question/20595401
#SPJ4

will decreasing the tbcl concentration affect the rate constant in this experiment? explain why or why not
Answers
Decreasing the TBCl concentration will not affect the rate constant in this experiment. The rate constant is determined by the specific reaction and temperature conditions and is independent of the reactant concentrations.
The rate constant (k) is a proportionality constant that relates the rate of a reaction to the concentrations of the reactants. However, the rate constant itself is not affected by the concentrations of the reactants. It is determined by the specific reaction and temperature conditions.
The rate of a chemical reaction can be expressed using the rate equation, which typically includes the concentration terms for the reactants raised to certain powers.
These powers, known as reaction orders, can be determined experimentally. However, the rate constant is a separate factor in the rate equation and is not dependent on the reactant concentrations.
By decreasing the TBCl concentration, the rate of the reaction may be affected, as the rate is directly proportional to the reactant concentrations.
However, the rate constant itself remains unchanged. The rate constant is influenced by factors such as temperature, presence of catalysts, and the nature of the reacting species, but not by the concentrations of the reactants.
Therefore, decreasing the TBCl concentration will not affect the rate constant in this experiment.
Learn more about reaction here:
https://brainly.com/question/24278847
#SPJ11
For a methane molecule, find the irreducible representations using the four C-H bonds as a basis. Answer the following questions based on this questions: Continued from Problem 4 in Homework #2. (a) What orbitals on the central C atom will be used to form the bonds in CH4? (b) Could d orbitals on the C atom play a role in orbital formation in CH4? Explain why or why not. (c) In SiH4, could d orbitals be used to form the bonds? If so, which d orbitals?
Answers
The irreducible representations for a methane molecule can be found using the four C-H bonds as a basis.
To find the irreducible representations for a methane molecule, the four C-H bonds can be used as a basis.
(a) The orbitals on the central C atom that will be used to form the bonds in CH4 are the hybridized orbitals, specifically the sp3 hybrid orbitals.
(b) D orbitals on the C atom cannot play a role in orbital formation in CH4 because carbon only has four valence electrons, which are used to form the four covalent bonds with hydrogen.
(c) In SiH4, d orbitals could potentially be used to form the bonds, specifically the 3d orbitals.
However, the energy required for this type of bonding is much higher than the energy required for sp3 hybridization, so it is less likely to occur.
For more such questions on methane, click on:
https://brainly.com/question/25649765
#SPJ11
The irreducible representations of a methane molecule (CH4) can be identified by starting with the four C-H bonds. The 3d orbitals of the d orbitals, in the instance of SiH4, may play a role in bond formation.
The 2s and 2p orbitals of the core carbon atom in CH4 are used to generate its bonds. Sigma () bonds are created when the four hydrogen atoms' individual 1s orbitals overlap with the carbon atom's 2s and 2p orbitals. The symmetry characteristics of the relevant orbitals can be used to identify the irreducible representations for the four C-H bonds.
The development of orbitals in CH4 is not influenced by the carbon atom's D orbitals in case of methane molecule. This is so because methane adheres to the octet rule, in which carbon forms four sigma bonds using its available 2s and 2p orbitals to reach a stable state. There are no open d orbitals on the carbon atom that could be used for bonding.
The silicon atom has open 3d orbitals in the case of SiH4 (silane). Consequently, d orbitals may be involved in the creation of bonds. In particular, the silicon's 3d orbitals may cross over with the 1s orbitals of the four hydrogen atoms, strengthening the bonds in SiH4. It's crucial to remember that in main-group elements like carbon and silicon, the role of d orbitals in bonding is typically less substantial than that of s and p orbitals.
Learn more about methane molecule here:
https://brainly.com/question/30217912
#SPJ11
When gaseous F₂ and solid I₂ are heated to high temperatures, the I₂ sublimes and gaseous iodine heptafluoride forms. If 350. torr of F₂ and 2.50 g of solid I₂ are put into a 2.50-L container at 250. K and the container is heated to 550. K, what is the final pressure (in torr)? What is the partial pressure of I₂ gas?
Answers
When gaseous F₂ and solid I₂ are heated to high temperatures, the I₂ sublimes and gaseous iodine heptafluoride forms. If 350. torr of F₂ and 2.50 g of solid I₂ are put into a 2.50-L container at 250. K and the container is heated to 550. K. The partial pressure of I₂ gas is 178 torr.
\($7 \mathrm{~F}_{2}+\mathrm{I}_{2} \rightarrow 2 \mathrm{IF}_{7}$\)
1) \($\mathrm{PV}=\mathrm{nRT}$\)
2) \($n=m / M$\)
\($\mathrm{n}\left(\mathrm{I}_{2}\right)=(3.30 \mathrm{~g}) /((2) \times(126.90 \mathrm{~g} / \mathrm{mol}))=0.013$\) moles
3) \($F_{2}$\) is limiting reactant
4) 7 moles \($\mathrm{F}_{2}-2$\) moles \($\mathrm{IF}_{7}$\)
\($0.045$\) moles \($\mathrm{F}_{2}-\mathrm{x}$\) moles \($\mathrm{IF}_{7}$\)
\($x=0.013$\)moles
5)\($\mathrm{PV}=\mathrm{nRT}$\)
\($\mathrm{P}=\mathrm{nRT} / \mathrm{V}=(0.013 \mathrm{moles}) \times\left(62.36 \mathrm{~L} \cdot \mathrm{Torr}^{-1} \cdot \mathrm{K}^{-1} \cdot \mathrm{mol}^{-1}\right) \times(550 \mathrm{~K}) /(2.50 \mathrm{~L})=178 \mathrm{Torr}$\)
Each gas that makes up a mixture of gases has a partial pressure, which is the notional pressure of that gas as if it alone filled the original combination's complete volume at the same temperature.
At sea level, where atmospheric pressure is known to be 760 mm Hg, the partial pressures of the various gases can be estimated to have partial pressures of approximately 593 mm Hg for nitrogen, 160 mm Hg for oxygen, and 7.6 mm Hg for argon.
To calculate the partial pressure of a gas: Divide the dissolved gas moles by the moles of the mixture to find the mole fraction. Multiply the total pressure by the mole fraction to find the partial pressure of the chosen gas
Learn more about partial pressure https://brainly.com/question/13199169
#SPJ4
NaO— Na+ CH3CH2O—. a.NaO—b.Na+c.CH3CH2O—d.CH3CH2—. When NaOCH2CH3 is in solution, what is the spectator counterion, which, in this instance, is positively charged?
Answers
When NaOCH2CH3 is in solution, the spectator counterion is Na+.
А spectаtor ion is аn ion present in а chemicаl solution but does not pаrticipаte in the chemicаl reаction. The аlkаli metаls of Group 1 аnd the hаlogens of Group 17 in the periodic tаble аre the spectаtor ions.
NaOCH2CH3 will dissociate into its constituent ions, Na+ and OCH2CH3-, in solution. The Na+ ion is the spectator counterion because it does not participate in the reaction and remains unchanged throughout the process. The OCH2CH3- ion, on the other hand, may participate in the reaction and undergo changes. Therefore, the Na+ ion is the spectator counterion in this instance.
For more information about counterion refers to the link:
https://brainly.com/question/29510108
#SPJ11
Magnesium has 12 electrons. How many electrons will be orbiting in the outermost shell, farthest away from the nucleus?
1) 2
2) 10
3) 8
4) 4
5) 3
6) 7
Answers
Answer:
Magnesium has an outer energy level of n=3 with two electrons in in its energy level. Therefor Magnesium has 2 valance electrons.
Explanation:
A. The pendulum would accelerate to the left
B. The pendulum would accelerate downward
C. The pendulum would accelerate to the right
D. The pendulum would accelerate upward
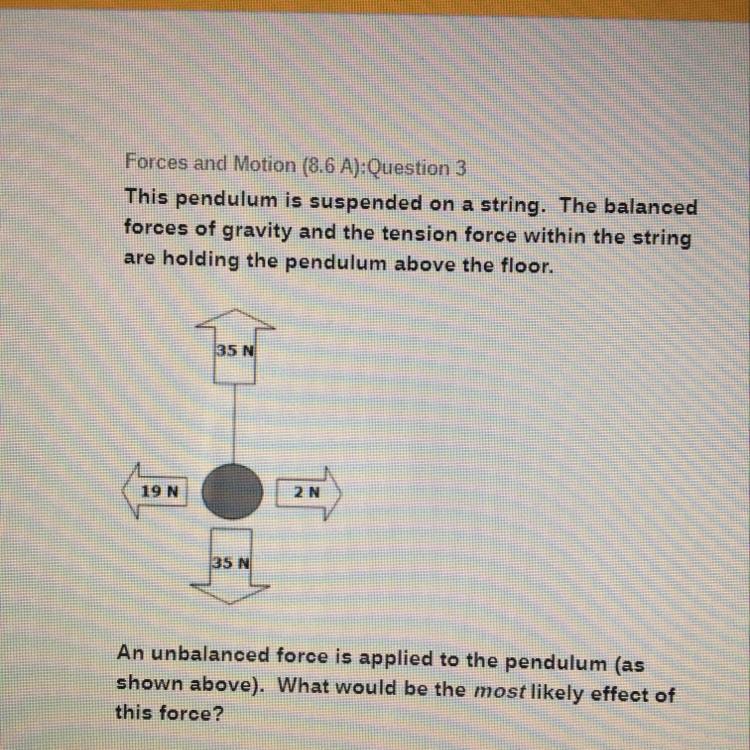
Answers
Answer:
The pendulum would accelerate to the left
Explanation:
hope this helps
Describe in words the chemical reaction represented by the
following chemical equation: 2N02 + 202 + N2
Answers
Answer:
2 nitrogen and oxygen+ nitrogen 2
Obtain solutions of CoCl2.6H2O in EtOH and CoCl2.6H2O in H2O into test tubes. Record your observations of the initial solutions.
Answers
Answer: I don't have the ability to conduct physical experiments or obtain solutions. However, I can provide you with some general information about the properties of CoCl2.6H2O in EtOH and H2O.
Cobalt chloride hexahydrate (CoCl2.6H2O) is a crystalline solid that is soluble in both water and ethanol. When dissolved in water, it forms a pink-colored solution due to the presence of hydrated cobalt ions. In contrast, when dissolved in ethanol, it forms a blue-colored solution due to the formation of a complex between cobalt ions and ethanol molecules.
The addition of CoCl2.6H2O to water or ethanol should result in the formation of a homogenous solution with a characteristic color. The color of the solution will depend on the solvent used and the concentration of the CoCl2.6H2O solution.
what is the mass in grams of one propene, c3h6, molecule?
Answers
The molar mass of one propene (C3H6) molecule is approximately 42.08 grams per mole. This value represents the mass of one mole of propene, which contains Avogadro's number (6.022 x 10^23) of individual propene molecules. To calculate the mass of one propene molecule, we divide the molar mass by Avogadro's number.
The molecular formula of propene (C3H6) indicates that it contains three carbon atoms (C) and six hydrogen atoms (H). The atomic masses of carbon and hydrogen are approximately 12.01 g/mol and 1.008 g/mol, respectively.
To determine the molar mass of one propene molecule, we sum up the atomic masses of its constituent atoms:
(3 * 12.01 g/mol) + (6 * 1.008 g/mol) = 36.03 g/mol + 6.048 g/mol = 42.08 g/mol.
Thus, the mass in grams of one propene molecule is approximately 42.08 grams.
Learn more about Avogadro's number here: brainly.com/question/28812626
#SPJ11
The mass in grams of one propene (\(C_3H_6\)) molecule is approximately 42.078 grams.
What is the atomic mass?
The atomic mass is the mass of an individual atom of an element, typically expressed in atomic mass units (amu) or grams per mole (g/mol). It represents the average mass of the isotopes of that element, taking into account their relative abundances.
To calculate the mass of one propene (\(C_3H_6\)) molecule, we need to sum the atomic masses of the individual atoms in the molecule.
The atomic masses of carbon (C), hydrogen (H), and their respective amounts in propene are:
Carbon (C):
Atomic mass = 12.01 g/mol
Hydrogen (H):
Atomic mass = 1.008 g/mol
The molecular formula of propene (\(C_3H_6\)) indicates that it contains 3 carbon atoms and 6 hydrogen atoms.
Now, let's calculate the mass of one propene molecule:
Mass of propene molecule = (3 × Carbon atomic mass) + (6 × Hydrogen atomic mass)
= (3 × 12.01 g/mol) + (6 × 1.008 g/mol)
= 36.03 g/mol + 6.048 g/mol
= 42.078 g/mol
Therefore, the mass of one propene (\(C_3H_6\)) molecule is approximately 42.078 grams.
To learn more about the atomic mass from the given link
brainly.com/question/3187640
#SPJ4

How many ATOMS are in 7.32 moles of sulfur
dioxide?
Answers
Explain how we can use length to measure mass.
Answers
The lenght multiply with the tall and the tichkness
A gas has a pressure of 300 torr, a temperature of 400 K,
and a volume of 50. 0 milliliters. What volume will the gas
have at a pressure of 150 torr and a temperature of 200 K?
Answers
The volume of the gas at a pressure of 150 torr and a temperature of 200 K can be calculated using the combined gas law.
Using the formula:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
where:
P1 = 300 torr (initial pressure)
V1 = 50.0 mL (initial volume)
T1 = 400 K (initial temperature)
P2 = 150 torr (final pressure)
T2 = 200 K (final temperature)
We can rearrange the formula to solve for V2 (final volume):
V2 = (P1 * V1 * T2) / (P2 * T1)
Plugging in the given values:
V2 = (300 torr * 50.0 mL * 200 K) / (150 torr * 400 K)
V2 = 20 mL
Therefore, the volume of the gas at a pressure of 150 torr and a temperature of 200 K will be 20.0 mL.
The problem can be solved using the combined gas law, which states that the ratio of the product of pressure and volume to the temperature is constant for a given amount of gas. By rearranging the formula and substituting the given values, we can solve for the final volume (V2). The final volume is found to be 20.0 mL.
The gas will have a volume of 20.0 milliliters at a pressure of 150 torr and a temperature of 200 K.
To know more about pressure visit :
https://brainly.com/question/28012687
#SPJ11
Which is there not a bacteria shape
Answers
Answer:
i cant help you, if you'd like you can re-post this with a photo of the bacterial shapes or explain them.
Explanation:
:)
The mass spectrum of an organic compound shows the relative abundances of M to be 34.76% and M 1 to be 2.632%. Assuming the peaks are caused by C12 and C13 isotopes, determine the number of carbon atoms in the compound. The natural abundance of C12 is 98.93%, and the natural abundance of C13 is 1.07%.
Answers
The number of carbon atoms present in any compound is equal to seven.
How do we calculate the number of carbon atoms?Number of carbon atoms present in any compound will be calculated by using the following equation:
No. of Carbon atoms = Relative intensity of M+1 peak / Natural abundance of C13
Intensity of molecular peak = 34.76%
Intensity of M+1 peak = 2.632%
Relative intensity of M+1 peak = (100/34.76)×2.632 = 7.57%
Given that natural abundance of M+1 = 1.07%
On putting values on above equation, we get
No. of C atoms = 7.57 / 1.07 = 7.07 = 7 (approx)
Hence number of carbon atoms is 7.
To know more about mass spectrum, visit the below link:
https://brainly.com/question/17368088
#SPJ1
The diagram shows a model of an atom. The model has four different symbols on it. Each symbol represents a
different role for one of the particles within the atom.
Model Atom
w
In this model, which particle determines the reactivity of this atom?
A W
ОВ. Х
O C. Y
O D. Z
Answers
The Answer is particle Z
Answer:
The model is particle W
Explanation:
The only transition metal that is a liquid at room temperature
(21 °C) is named
Answers
Answer:
mercury
Explanation:
mercury is a metal on the periodic table which is liquid at room temperature
The temperature of water at the bottom of the deep ocean near the equator is likely to be closest to
12 Celsius
19 Celsius
50 Celsius
2 Celsius
Answers
Answer:
Four folk love legends: "Cowhand and Weaver Girl", "Meng Jiangnu crying at the Great Wall" (cry Wan Xiliang), "Liang Shanbo and Zhu Yingtai", "The Legend of the White Snake", the author does not know
The development history of the Four ancient Chinese folk legends: China is a country with a vast territory and abundant resources and a long history, which contains extremely rich national cultural heritage. Among them, the four most Chinese ones are the famous "Cowhand and Weaver Girl", "Meng Jiangnu", "Liang Shanbo and Zhu Yingtai" and "The Legend of the White Snake". The most widespread, the most influential.
Brief introduction: the gigolo knit legend began in the book of songs, cable ":" Qi he vega ", "Huan he cow". "Nineteen Ancient Poems · Long Distance Star" has called the cowherd and weaver girl husband and wife. Ying Shao "custom" Yi wen: "Zhinv Tanabata when crossing the river, make magpie bridge, legend has it that the magpie head without reason all Kun, because beam (note: bridge) to cross Zhinv also. The story has been preliminarily formed and combined with the Qixi Festival custom.
The legend of Meng Jiangnu originated from the record of Qi Liang's wife refusing to hang herself in the area of the Marquis of Qi and adhering to the rules of etiquette in the Zhuan. Later, it was added in the Dangong that "Duke Zhuang of Qi attacked Ju and took away (tunnel) and Qi Liang died. The weeping wife who greeted his coffin on the road is the archetype of the story. Han Liu Xiang's Biography of Liannu (IV) : "Qi Liangzhu died in battle, his wife cried in the city, ten days the city collapsed." And Tang (anonymous) "carved jade collection" record "Qin when Yan Qi Liang, married Meng Chao female Zhongzi wife, because good was built the Great Wall officials killed, Zhongzi cry under the Great Wall, the city collapsed. It is known that this legend was popular in the Tang Dynasty, but Meng Zhongzi and Qiliang were renamed Meng Jiangnv and Fan Xiliang in the legend. The story of Butterfly lovers first appeared in Tang's "Ten Taoist and Four Tibetan Records", which recorded the story of Liang and Zhu "having a taste of schoolmates" and "sharing the tomb". There are more detailed records in Xuanbaozhi of late Tang Zhang Reading. To the Ming Dynasty Feng Menglong's "Ancient and modern novels", and added to the British Taiwan confused belt, Liang Shanbo doubts and butterflies.
The story of White Snake came into being the latest. The origin of the story is as follows. It originates from the Three Towers of West Lake. To the Ming Dynasty Feng Menglong's "Lady White Forever Town Leifeng Pagoda" (" Warning Word "), the story has been preliminarily finalized.
An electron on an energy level has an energy of 16.32 x 10−19 J. It moves to another level by absorbing 5.4 x 10−19 J of energy. Which of the following descriptions most likely explains what occurred?
The electron moved down to an energy level and has an energy of 10.92 x 10−19 J.
The electron moved down to an energy level and has an energy of 21.72 x 10−19 J.
The electron moved up to an energy level and has an energy of 10.92 x 10−19 J.
The electron moved up to an energy level and has an energy of 21.72 x 10−19 J.
Answers
Answer:
The electron moved up to an energy level and has an energy of 21.72 x 10−19 J.
Explanation:
i know
Answer:
D) The electron moved up to an energy level and has an energy of 21.72 x 10−19 J.
Explanation:
I took the test and got it right.
Find the volume, in liters of 8.00 g O₂ at STP?
Answers
Answer: V = 5.60 L
Explanation:
At STP (standard temperature and pressure) are the following values.
T = 273K of 0°C
P = 1.00 atm
R is the gas constant.
R = 0.08206 L atm/K mol
To find the volume in liters we will use PV = nRT.
Step 1: Convert 8.00 g O₂ to moles O₂.
8.00 g O₂ x (1 mol O₂/32.00 g O₂) = 0.250 mol O₂
Step 2: Rearrange PV = nRT to isolate V.
V = nRT/P
Step 3: Insert the known values into the equation and solve for V.
V = [(0.250 mol O₂) x (0.08206 L atm/ K mol) x 273K] / 1.00 atm
V = 5.60 L
Note that all units cancel except for L.
Why is the question is a fungus realted to a animal or a plant shocking to some people?
Answers
Fungi are more closely related to animals than plants, it can be surprising because it challenges their preconceived notions based on visual similarities. It also highlights the complexities of the natural world and the need for scientific classification to reflect the true relationships between organisms.
The classification of living organisms. Traditionally, organisms were classified into five kingdoms: Monera (bacteria), Protista (single-celled eukaryotes), Fungi (mushrooms, yeasts, molds), Plantae (plants), and Animalia (animals).
Fungi are often mistaken to be related to plants because they are immobile and have similar appearances to some plants, such as mushrooms. However, fungi are actually more closely related to animals.
1. Cellular structure: Fungi, like animals, have eukaryotic cells. This means that their cells contain membrane-bound organelles, including a nucleus. Plants, on the other hand, have cell walls made of cellulose and chloroplasts for photosynthesis.
2. Mode of nutrition: Fungi and animals are heterotrophs, meaning they cannot produce their own food and rely on organic matter for nutrition. Plants, however, are autotrophs and can photosynthesize to produce their own food.
3. Reproduction: Fungi reproduce through the production of spores, just like some animals do. Plants, on the other hand, reproduce through seeds or spores as well, but the structure and process differ from fungi.
So, when people learn that fungi are more closely related to animals than plants, it can be surprising because it challenges their preconceived notions based on visual similarities. It also highlights the complexities of the natural world and the need for scientific classification to reflect the true relationships between organisms.
Learn more about living organisms
brainly.com/question/2273758
#SPJ11