Answers
Answer
The empirical formula for this compound PbN₂O₆Explanation
Given:
% composition: 62.6% Pb, 8.4% N, and 29% O
What to find:
The empirical formula for the compound
Step-by-step solution:
Assume we have 100 grams total of the substance.
Grams Pb: 62.6 g
Grams N: 8.4 g
Grams O: 29 g
Now, let's convert the number of grams of each element into moles below:
Moles Pb: 62.6 g/207.2 g/mol = 0.30 mol
Moles N: 8.4 g/14.0 g/mol = 0.60 mol
Moles O: 29 g/16.0 g/mol = 1.81 mol
Now, let's divide the number of moles of each element by the smallest number obtained:
Pb = 0.30/0.30 = 1
N = 0.60/0.30 = 2
O = 1.81/0.30 = 6
Therefore the empirical formula for this compound PbN₂O₆Related Questions
Water boils at 100 degrees Celsius. What is the boiling point for water on the Kelvin scale? K = °C + 273 and °C = K - 273
Answers
Answer:
the answer is 373
Explanation:
K=°C + 273
K=100+273=373
How many moles of CaSO4 are required to produce 128 g of SO2? 3CaSO4 + CaS → 4CaO + 4SO2
Answers
2 moles of CaSO4 are required to produce 128 g of SO2 in 3CaSO4 + CaS → 4CaO + 4SO2 in this equation.
What do you mean by mole ?The term mole is defined as a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
1 mole = 6.023 × 10²³ molecules
The balance equation is as follows:
3CaSO4 + CaS → 4CaO + 4SO2
1 mole = 6.023 × 10²³ molecules
1 mole SO₂ = 6.023 × 10²³ molecules
128 mole = ?
Molar mass of SO₂ = 64 gram
The numbers of moles in 128 gram SO₂ = 1 / 64 ×128
= 2 moles
Thus, 2 moles of CaSO4 are required to produce 128 g of SO2.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Bond energy of amphetamine?
Answers
Answer:
Amphetamine | C9H13N | CID 3007 - structure, chemical names, physical and chemical properties , ... Defined Bond Stereocenter Count, 0, Computed by PubChem ... Collision Energy, 10 eV .
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
Experiments with cathode rays being deflected by a magnetic field
show that cathode rays are composed of particles that are
positively charged or negatively charged
Answers
Answer:
Negatively charged
Explanation:
Answer:
Negatively charged
Explanation:
The graph in part A shows how slow-moving water cannot carry large pieces of sediment. As water slows down on the inside of a curve, some of the larger pieces of sediment will settle out of the water. What effect will the settling of sediment have on the shape of the stream?
Answers
The river will deposit sediment along the inside of a bend in the river. This deposition causes the inside of the curve to become shallower and move farther inward.
What are Meandering Rivers ?Rivers flowing over gently sloping ground begin to curve back and forth across the landscape. These are called meandering rivers.
Meandering rivers erode sediment Larry from the outer curve of each meander bend and deposit it on an inner curve further down stream.
This causes individual meanders to grow larger and larger over time.
Meandering river channels are asymmetrical. The deepest part of the channel is on the outside of each bend. The water flows faster in these deeper sections and erodes material from the river bank.
The water flows more slowly in the shallow areas near the inside of each bend. The slower water can't carry as much sediment and deposits its load on a series of point bars.
Thus the settling of sediment will make the stream steeper.
To know more about Meandering Rivers
https://brainly.com/question/7560368
#SPJ1
Answer:
The river will deposit sediment along the inside of a bend in the river. The deposition causes the inside of the curve to become shallower and move farther inward.
Explanation:
Edmentum
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
3: Given 12.3 grams of NH3, how many moles of N₂ were needed?
Answers
0.361 moles of N₂ were required to produce 12.3 g of NH₃, using the balanced chemical equation N₂ + 3H₂ → 2NH₃.
The balanced chemical equation for the reaction is N₂ + 3H₂ → 2NH₃. We can use the balanced equation and the molar mass of NH₃ to calculate the number of moles of N₂ required to produce 12.3 g of NH₃,
1 mol NH₃ = 2 mol N₂ (from the balanced equation)
molar mass of NH₃ = 17.03 g/mol
moles of NH₃ = 12.3 g / 17.03 g/mol
moles of NH₃ = 0.722 mol
moles of N₂ = (0.722 mol NH₃) / 2
moles of N₂ = 0.361 mol
Therefore, 0.361 moles of N₂ were needed to produce 12.3 grams of NH₃.
To know more about moles, visit,
https://brainly.com/question/29367909
#SPJ1
Complete question - For the reaction, N₂ + 3H₂ → 2NH₃. Given 12.3 grams of NH3, how many moles of N₂ were needed?
Determine the molecular formula for a compound with
an empirical formula of C2h8N and a molar mass of 46
grams per mole?
Answers
Answer:
C2H8N
Explanation:
Hello there!
In this case, since the molecular formula is the complete representation of the considered chemical compound and the empirical one is the smallest possible whole-numbered representation of the former, we infer that the ratio of the molar masses of these two provides the times in which the empirical formula is within the molecular one; for that reason, we first compute the molar mass of the empirical formula:
\(MM_{empirical}=12*2+8+14=46g/mol\)
Thus, since the ratio is 46/46=1, we infer that the molecular formula is also C2H8N.
Best regards!
Methyl isocyanate, shown as resonance structure 1, can also be represented by other resonance structures. Draw the next most important resonance contributor. Then add curved arrows to each structure to show delocalization of electron pairs to form the other structure.
Include lone pairs of electrons, formal charges, and hydrogen atoms. You can add condensed hydrogens using the More menu, selecting +H and clicking on the carbon as many times as needed.
Answers
Solution :
Structure I
The formal charge on both Carbon (C) atom is = 4 valance \($e^-$\) - bonds = 0
Formal charge (O) = 6 V.E - 2 bonds - 4 non bonding electrons = 0
Formal charge on (N) = 5 V.E - 3 bonds - 2 non bonding electrons = 0
F.C. on H = 1 V.E. - 1 bond = 0
Overall charge on the molecule = 0 charge
Structure II
Formal charge on both C atom = 4 valence \($e^-$\) - 4 bonds = 0
Formal charge (O) = 6 V.E. - 1 bonds - 6 non bonding electrons = -1 charge
Formal charge on (N) = 5 V.E. - 4bonds - 0 non bonding electrons = +1 charge
F.C on H = 1 V.E. - 1 bond = 0
Overall charge on the molecule = +1 -1
= 0 charge

You add 0.59 mL of n-propanol (0.803 g/mL, 60.1 g/mol) to an excess of acetic acid to create the pear ester propyl acetate (102.1 g/mol). If you obtain 0.54 grams of the product, what was your percent yield
Answers
The percentage yield of the reaction between n-propanol and excess of acetic acid is 67.5%
How to determine the mass of n-propanolVolume = 0.59 mL Density = 0.803 g/mLMass of n-propanol =?Mass = Density × Volume
Mass of n-propanol = 0.803 × 0.59
Mass of n-propanol = 0.47 g
Balanced equationSee attached photo
Molar mass of n-propanol = 60.1 g/mol
Mass of n-propanol from the balanced = 1 × 60.1 = 60.1 g
Molar mass of propyl acetate = 102.1 g/mol
Mass of proply acetate from the balanced equation = 1 × 102.1 = 102.1 g
SUMMARY
From the balanced equation,
60.1 g of n-propanol reacted to produce 102.1 g of propyl acetate
How to determine the theoretical yieldFrom the balanced equation,
60.1 g of n-propanol reacted to produce 102.1 g of propyl acetate
Therefore,
0.47 g of n-propanol will react to produce = (0.47 × 102.1) / 60.1 = 0.8 g of propyl acetate
How to determine the percentage yield Actual yield of propyl acetate = 0.54 gTheoretical yield of propyl acetate = 0.8 gPercentage yield =.?Percentage yield = (Actual / Theoretical) × 100
Percentage yield = (0.54 / 0.8) × 100
Percentage yield = 67.5%
Learn more about stoichiometry:
https://brainly.com/question/14735801

Which type of bonding involves the sharing of electrons, in which the electrons are confined close to the more electronegative element in the bond?
Question 1 options:
Covalent bonding
Metallic bonding
Ionic bonding
Polar covalent bonding
Answers
Answer:
metallic bonding
because it ids good conductor of electricity
Answer:
Covalent Bonds
A or B or C or D, plz answer fassttt

Answers
Answer:
A.
Explanation:
They'd probably want to know the conditions that usually produce fog
What is the boiling point in °C of a 3.6 molal solution of calcium chloride in water?

Answers
Answer:
CaCl2--->Ca2+ + 2Cl_ so i=3
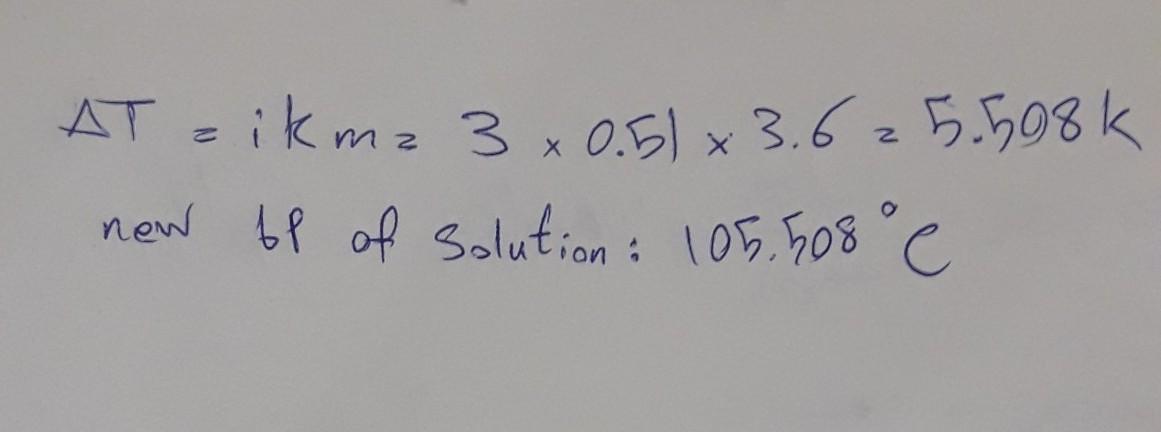
The oxides SO2 and N2O5 will form what acids?
Answers
SO2 + H2O → H2SO3
N2O5, on the other hand, reacts with water to form nitric acid (HNO3), which is a strong acid. The reaction can be represented as follows:
N2O5 + H2O → 2HNO3
Therefore, SO2 forms sulfurous acid (H2SO3) and N2O5 forms nitric acid (HNO3) when they react with water.
With the following data, construct a heating curve for the substance. Show relative differences and proper labels.normal melting point: 20 degrees celsius molar heat of fusion: 3KJ/molnormal boiling point: 126 degrees celsiusmolar heat of vaporization: 80KJ/mol
Answers
A heating curve gives us information on how much energy is required to change the state of matter of a substance and at what temperature this change occurs.
The x-axis represents the heat to be added and the y-axis the temperature. On the graph, when a phase change occurs, the temperature will remain constant, obtaining a straight line.
The melting point is the temperature at which a substance changes from a solid to a liquid state. The melting point will be linked to the molar heat of fusion.
The boiling point is the temperature at which there is a change of state from liquid to gas, the boiling point is linked to the molar heat of vaporization.
The heat curve for this case will be:

Taiga forests are ________ and receive ________ rainfall than temperate broadleaf forests.
colder, less
warmer, more
warmer, less
colder, more
Answers
Answer:
1st one
Explanation:
Answer:
Colder, less
Explanation:
I did the test
For the reaction 2 H2(g) + O2(g) → 2 H2O(g), how many liters of water can be made from 5.0 L of
oxygen gas and an excess of hydrogen?
Answers
Answer: 5L O2 x 1 mol O2/ 22.4L O2 x 2 mol H20/ 1 mol O2 x 22.4L H20/ 1 mol H20 = 10 L H2O
Explanation:
For future reference though, since its at STP that means that the coefficient are in proportion. Since oxygen has a coefficient of 1 and water has a coefficient of 2 for every 1 liter of oxygen there is 2 liters of water. Hence you started with 5 liters and ended with 10
Which of the following is converted into chemical energy during photosynthesis?
A
oxygen
B
sound energy
с
light energy
D
glucose
Answers
Which refers to the passing of a wave through an object?
sound
O interference
O transmission
O frequency
O sound
Answers
The term that refers to the passing of a wave through an object is "transmission."
Transmission refers to the process by which a wave passes through an object or medium. In the context of sound, transmission occurs when sound waves travel through different substances, such as air, water, or solids.
When a sound wave encounters an object, it can be transmitted through it, reflected off it, or absorbed by it, depending on the properties of the object and the medium through which the sound is traveling.
For example, when you speak into a microphone, the sound waves produced by your voice travel through the air and are transmitted to the microphone's diaphragm. The diaphragm converts the sound waves into electrical signals, which can then be amplified and reproduced as sound through speakers.
In summary, transmission is the term used to describe the passage of a wave, such as a sound wave, through an object or medium. It is an essential concept in understanding how waves interact with their surroundings and how sound propagates through different materials.
for such more questions on transmission
https://brainly.com/question/18451537
#SPJ8
Determine weather each of the following will be saturated or unsaturated at 50 degree celcious
Answers
The solubility of the solutes in the solution will determine whether each of the solutions will be saturated or unsaturated at 50 degree celcius.
What are saturated and unsaturated solutions?Saturated solutions are solutions which contains as much solute as they can dissolve at that temperature in the presence of undissolved solute.
An unsaturated solution is a solution which contains less solute than it can dissolve at that temperature.
The solubility of a solute determines whether its solution will be saturated or unsaturated at a given temperature.
Therefore, the solubility of the solutes in the solution will determine whether each of the solutions will be saturated or unsaturated at 50 degree celcius.
Learn more about saturated and unsaturated solutions at: https://brainly.com/question/15462976
#SPJ1
Bromine trifluoride’s free-energy change is –229.4 kJ/mol, and water vapor’s is –228.6 kJ/mol. Though their free-energy changes are almost the same, bromine trifluoride reacts violently with water vapor, releasing much more energy than the water vapor. The field of chemistry called ______________ explains why bromine trifluoride reacts one way and water vapor reacts another during a reaction.
Answers
The field of chemistry that explains why bromine trifluoride reacts differently than water vapor during a reaction is called chemical kinetics.
What is Energy?
Energy is a fundamental concept in physics, often defined as the ability to do work. It can take many different forms, including thermal energy, kinetic energy, potential energy, electromagnetic radiation, and chemical energy, among others. Energy can be transferred from one system to another, and it can also be converted from one form to another. The SI unit of energy is the joule (J), although other units such as the calorie and the electronvolt are also used in specific contexts.
The field of chemistry that explains why bromine trifluoride reacts differently than water vapor during a reaction is called reaction kinetics. Reaction kinetics is the study of how fast chemical reactions occur and what factors affect the reaction rate. It takes into account factors such as the nature of the reactants, concentration, temperature, surface area, and the presence of a catalyst or inhibitor.
Learn more about Energy
https://brainly.com/question/2003548
#SPJ1
Chemistry
Help me please.

Answers
Answer:
4:6
Explanation:
Which is the correct electron-dot formula for the molecule chlorine?

Answers
Answer: It is option 4
Explanation:
The Chlorine have to share 2 electrons
Where does photosynthesis occur in a plant cell, specifically what
organelle?
2 points
Your answer
During the chemical reaction of photosynthesis. Light energy is converted 2 points
into what type of energy?*
Your answer
2 points
Look at this image, which numbers represent the PRODUCTS of
photosynthesis?
Sun's energy
Answers
Answer:
1) chloroplast
A chloroplast is an organelle within the cells of plants and certain algae that is the site of photosynthesis, which is the process by which energy from the Sun is converted into chemical energy for growth.
2)Photosynthesis is the process in which light energy is converted to chemical energy in the form of sugars. In a process driven by light energy, glucose molecules (or other sugars) are constructed from water and carbon dioxide, and oxygen is released as a byproduct.
3)i need the photo to answer
Explanation:
PLEASE MARK ME AS BRAINLIEST
5. What physical property of foam makes it a good choice to
use in life vests?
A. Foam has a relative density less than water.
B. Foam is a good thermal insulator.
C Foam is non-magnetic.
D. Foam is soluble in water.
Answers
Fun With Predicting Reaction Products
I erased my answers so far but i’m confused about every thing on this paper. Pleaseeeee help thanks

Answers
To predict the products of such a reaction, see what happens if the chemical breaks into smaller, familiar products such as water, carbon dioxide, or any of the gaseous elements
Describe the type of reaction that was indicated.AgNO3+Na2SO4→AgSO4+2NaNO3 is an exchange reaction.
Na + O2→ Na2O .
is an exchange reaction.
Thus, we refer to these processes as redox. Na is oxidized by losing electrons in reaction (a), while O is reduced by gaining electrons, forming O2-ions.
A single-displacement reaction would be Mg + HBr > MgBr2 + H2 HBr + Mg. In this process, magnesium creates magnesium bromide by swapping out hydrogen from HBr (MgBr2).
The decomposition process CuSO4(aq) + Zn(s) ZnSO4(aq) + Cu(s) is an illustration.
To learn more about reaction refers to:
https://brainly.com/question/11231920
#SPJ1
1.
For each of the ions listed, identify the total number of electrons for each
1. Al+3
2. Fe¹3
3. Mg²
4. Sn¹²
5. Co²
6. Co³
7. Lit¹
8. Cr+3
9. Rb¹
10. Pt+2
Answers
The total number of electrons Al+3. Fe¹3 Mg² Sn¹² Co²Co³ Lit¹ Cr+3, Rb Pt+2 are 3, 3, 2, 12, 2, 3, 0, 3.0,2 electrons
Electrons calculations explained.
Electrons are subatomic particles that have a negative charge and are found outside the nucleus of an atom. They orbit the nucleus in shells or energy levels and are involved in chemical bonding and electricity. Electrons are much smaller in size compared to protons and neutrons, which make up the nucleus of an atom.
Al+3 has 10 electrons (13 protons - 3 electrons).
Fe¹3 has 20 electrons (26 protons - 3 electrons).
Mg² has 10 electrons (12 protons - 2 electrons).
Sn¹² has 50 electrons (50 protons - 12 electrons).
Co² has 27 electrons (27 protons - 2 electrons).
Co³ has 26 electrons (27 protons - 3 electrons).
Li¹ has 3 electrons (3 protons - 0 electrons).
Cr+3 has 21 electrons (24 protons - 3 electrons).
Rb¹ has 37 electrons (37 protons - 0 electrons).
Pt+2 has 76 electrons (78 protons - 2 electrons).
Therefore, The number of electrons in an atom determines its chemical behavior and the way it interacts with other atoms.
Learn more about electrons below.
https://brainly.com/question/26084288
#SPJ1
The volume of a gas is 325 mL when the temperature is 57°C. If the temperature is reduced to 10°C without changing the pressure, what is the new volume of the gas? Combined gas law 2 P₁V₁ T₁ P₂V2 T2 = 278.7 mL
Answers
Answer:
The new volume of the gas is 278.7 mL.
Explanation:
To solve this problem, we can use the combined gas law:
P₁V₁/T₁ = P₂V₂/T₂
where P is the pressure, V is the volume, and T is the temperature.
We can start by plugging in the given values for the initial state of the gas:
P₁ is not given, so we can assume it remains constant.
V₁ = 325 mL
T₁ = 57°C + 273.15 = 330.15 K
Now we can solve for P₂V₂/T₂:
P₂V₂/T₂ = P₁V₁/T₁
We want to solve for V₂, so we can rearrange the equation:
V₂ = (P₁V₁/T₁) * T₂/P₂
We are given that the pressure remains constant, so P₁ = P₂.
Now we can plug in the remaining values:
V₂ = (P₁V₁/T₁) * T₂/P₂
V₂ = (P₁ * 325 mL / 330.15 K) * (10°C + 273.15) / P₁
V₂ = 278.7 mL
Therefore, the new volume of the gas is 278.7 mL.
Hope I helped you!