Answers
Answer: The missing component in the nuclear equation is:
231 Th → 90 B + X + 91 He
where X represents the missing component.
In this equation, thorium-231 (231 Th) undergoes radioactive decay and transforms into bismuth-90 (90 B), a particle (represented by X), and helium-4 (91 He). The mass number of the missing component, X, can be calculated by subtracting the mass numbers of bismuth-90 and helium-4 from the mass number of thorium-231:
X = 231 Th - 90 B - 91 He
= 231 - 90 - 91
= 50
The atomic number of the missing component, X, can be calculated by subtracting the atomic numbers of bismuth-90 and helium-4 from the atomic number of thorium-231:
X = 231 Th - 90 B - 91 He
= 90 - 83 - 2
= 5
Based on these calculations, the missing component X is likely to be vanadium-50 (50 V). This is a reasonable prediction because vanadium is a naturally occurring element that is known to undergo radioactive decay, and it has an atomic number of 23 and a mass number of 50. However, it's important to note that this prediction is based on the assumption that the nuclear equation is balanced, which means that the number of protons and neutrons on both sides of the equation is the same. If the equation is not balanced, then the missing component X could be something else.
Related Questions
If an ideal gas has a pressure of 1.71 atm, a temperature of 68.16 ∘C, and a volume of 12.85 L how many moles of gas are in the sample?
Answers
Answer:
0.745 moles
Explanation:
We can use the ideal gas law, which relates the pressure (P), volume (V), number of moles (n), and temperature (T) of a gas:
P V = n R T
where R is the gas constant.
We can rearrange this equation to solve for n:
n = (P V) / (R T)
We can look up the value of the gas constant for units of atm L / (mol K). The value is approximately 0.08206 (atm L) / (mol K).
Substituting the given values, we get:
n = (1.71 atm) * (12.85 L) / (0.08206 (atm L) / (mol K) * (68.16 + 273.15) K)
where we have converted the temperature from Celsius to Kelvin by adding 273.15.
Evaluating this expression gives us:
n ≈ 0.745 mol
Therefore, there are approximately 0.745 moles of gas in the sample.
The rate law of the overall reaction A + B → C is rate = k[A]2. Which of the following will not increase the rate of the reaction?
a. increasing the concentration of reactant B
b. adding a catalyst for the reaction
c. increasing the concentration of reactant A
d. increasing the temperature of the reactant
e. all of these will increase the rate
Answers
To solve this we need to have knowledge of experimental rate law which relates the rate to the concentration raise to some power. Therefore, the correct option is option A that is increasing the concentration of reactant B.
What is rate law?A rate law represents the rate of a reaction . According to this rate is directly proportional to the concentration of reactants. There is another expression of rate law which is integrated rate law which is just opposite of differential rate law.
The experimental rate law for given reaction is given as:
Rate = rate = k[A]²
To not to increase the rate of the reaction, we need to increasing concentration of B as there is no concentration of B in the rate law expression. Rate is independent of concentration of B.
Therefore, the correct option is option A that is increasing the concentration of reactant B.
To know more about rate law, here:
https://brainly.com/question/14779101
#SPJ5
Which of the following are diatomic elements? Select all that apply.
A.Hydrogen
B.Boron
C.Bromine
D.Iodine
Answers
Answer:
C
Explanation:
A diatomic element in that list is Bromine
Which are ways to ensure that temperature readings are accurate? Check all that apply. shaking the thermometer before use using the thermometer as a stirring rod making sure the liquid inside the thermometer is at eye level when taking the temperature making sure the bulb of the thermometer does not touch the bottom of the beaker or the ice when taking the temperature using the Celsius scale instead of the Fahrenheit scale when taking temperature readings
Answers
Answer:
Explanation:
making sure the liquid inside the thermometer is at eye level when taking the temperature
making sure the bulb of the thermometer does not touch the bottom of the beaker or the ice when taking the temperature
Describes the chemical reaction (s) that produce AMD. Equations
are balanced and formatted to show subscripts.
Pls help I’m so confused
Answers
FeS2 + 7O2 + H2O → Fe2+ + 2SO4^2- + 2H+
This reaction is an oxidation reaction, where the sulfide mineral is oxidized to sulfate ions and ferrous ions are released. The ferrous ions can then react with water and oxygen to form ferric hydroxide (Fe(OH)3), which is a yellow-orange solid that contributes to the characteristic color of AMD.
The overall reaction can be written as:
4FeS2 + 15O2 + 14H2O → 4Fe(OH)3 + 8SO4^2- + 16H+
This reaction shows that four molecules of pyrite react with 15 molecules of oxygen and 14 molecules of water to produce four molecules of ferric hydroxide, eight molecules of sulfate ions, and 16 molecules of hydrogen ions. The reaction is balanced to ensure that the number of atoms of each element is the same on both sides of the equation.
Some automobiles and buses have been equipped to burn propane (C_3H_8). Compare the amounts of energy that can be obtained per gram of C_3H_8(g) and per gram of gasoline, assuming that gasoline is pure octane, C_8H_18(l). (See Example 6.11.) Look up the boiling point of propane.
Answers
Propane will release a higher amount of energy per gram than octane.
The known heat of combustion for propane is −50.3kJ/g which means that one gram of the hydrocarbon will release or give -50.3 kJ of energy. On the other hand, the heat of combustion for octane is −47.9kJ/g. The heat of combustion for octane tells us that if we burn one gram of octane, it will give 47.9 kJ of energy.
Based on the values we provide for the question, propane will release a higher amount of energy per gram than octane.
What is energy?
Common forms of energy include kinetic energy of a moving object, potential energy stored by an object (for example due to its position in a field), elastic energy stored in a fixed object, chemical energy associated with chemical reactions, radiation. energy carried by electromagnetic radiation and internal energy contained in a thermodynamic system. All living organisms constantly receive and release energy.To know more about energy, click the link given below:
https://brainly.com/question/29763772
#SPJ4
what would the result be of adding one proton to an atom
Answers
Answer:Protons carry a positive electrical charge and they alone determine the charge of the nucleus. Adding or removing protons from the nucleus changes the charge of the nucleus and changes that atom's atomic number. For example, adding a proton to the nucleus of an atom of hydrogen creates an atom of helium and thats your answer
Explanation:
yurrrrrrr
O Macmillan Learning
What is the IUPAC name for the compound shown?
IUPAC name:
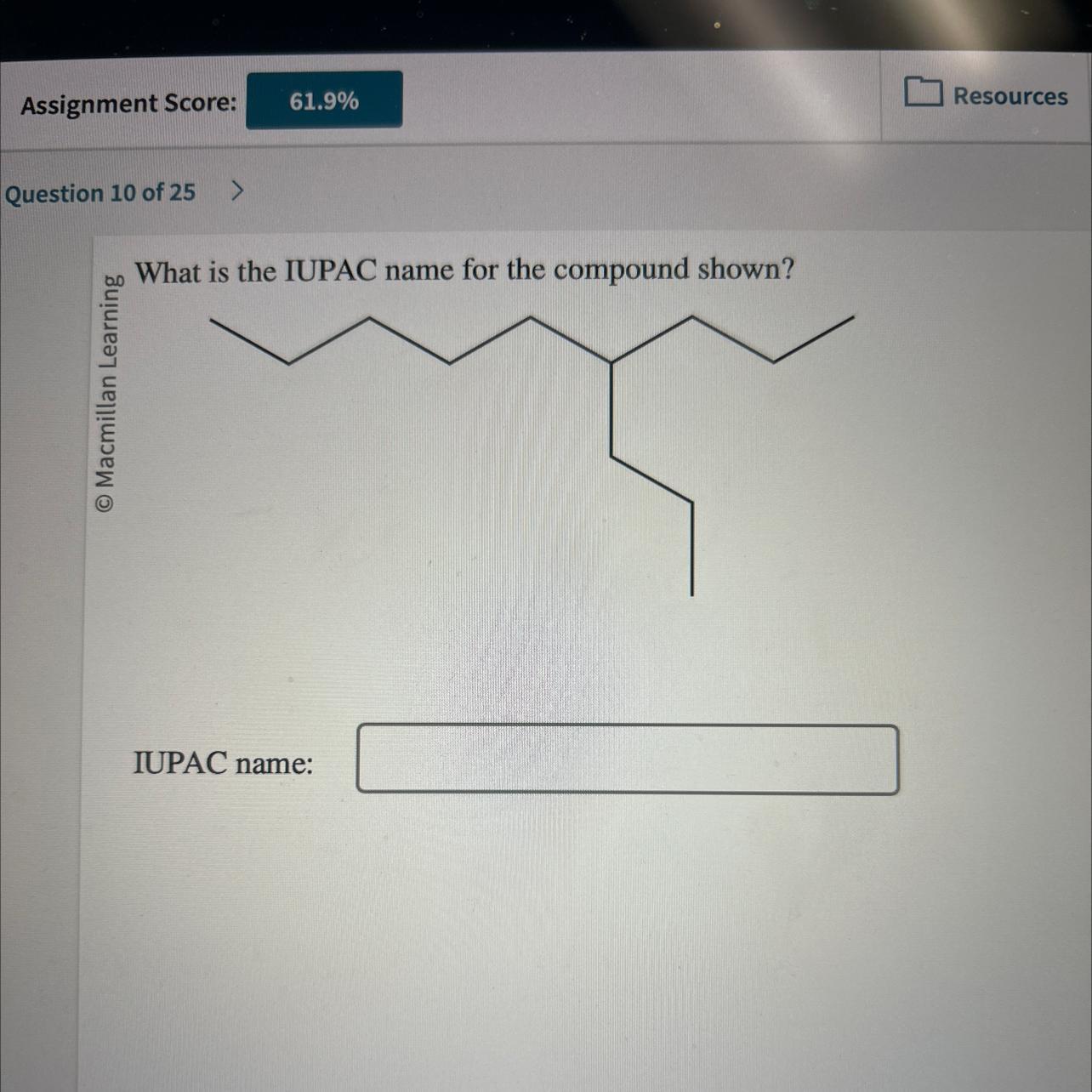
Answers
The compound shown has the IUPAC name O Macmillan Learning. -3-ethyl-2,2-dimethyl-hexane Incorrect.
Why was 1 6 dimethylhexane wrong?Explanation and response: Because it implies that there's methyl groups on carbons number one and sixth of the parent carbon chain, the name — appears-dimethylhexane is incorrect. Because the name "hexane" implies that a parent atom chain only has six molecules long, the methyl groups are located at the ends of every molecule.
Is hexane considered an organic chemical?Hexane, commonly referred to as sextane, is an organic compound that belongs to the alkane class. They are acyclic branched as well unbranched hydrocarbons with the standard structure CnH2n+2, and thus entirely composed of hydrogen and saturated oxygen atoms. Hexane is a colorless, clear liquid.
To know more about dimethylhexane visit :
https://brainly.com/question/30438544
#SPJ1
Common human behavior that seems to be programmed or directed by genes or hormones?
Answers
Answer:
There's a lot...lying...rolling eyes...aggression...mating...parenting...sadness...basically alot
Explanation:
which law states that the pressure and absolue tempeture of a fixed quantity of gas are directly proportional under constant volume conditions?
Answers
Answer:
Gay lussacs law
Explanation:
HELP!!!!!!
I DON'T KNOW THE ORDER!!

Answers
Answer:
for 1 solid its freezing.
for 2 solid and liquid its melting
for 6 liquid to gas its evaporation and for 5 gas to liquid its condensation.
Explanation:
hope this helped :)
Answer:
solid->liquid= melting
liquid->solid= freezing
gas->liquid= consendation
liquid->gas= evaporation
Part A Identity the reactants and products in this chemical equation Drag the appropriate labels to their respective targets. Reset Help reactant 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(8) product
Answers
In the reaction: 4 NH₃ + 5 O₂ → 4 NO + 6 H₂O, the reactants are NH₃ and O₂ and the products are NO and H₂O.
The balanced chemical reaction is given as,
4 NH₃ + 5 O₂ → 4 NO + 6 H₂O
The substance present in the left of the arrow in a chemical equation are called reactants. Basically, a reactant is a substance that is present at the start of a chemical reaction. The substance(s) present in the right of the arrow are called products. Basically, a product is defined as a substance that is present at the end of a chemical reaction.
Learn more about chemical reaction from the link given below.
https://brainly.com/question/29039149
#SPJ4
Nobody answering my questions what the answer givng brainliest

Answers
Answer: I think it’s B. Because as you can see from the picture there are layers
Explanation:
I think but just in case ask for a second opinion
Answer:
C. Air bubbles
Explanation:
Cant be A because.... you cant freeze a tempreature
Cant be B because... organism types cant show precipitation patterns.
Cant be D because....well it could be D, but there isnt a way to prove humans increased the tempreature
ITS C hope it helps :)
I also got this question before
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
CaCO3(s)– Cao(s) + CO2(g)
AGf.Cacoa = -1,128.76 kJ/mol
AGf, Cao = -604.17 kJ/mol
AGT,CO, = -394.4 kJ/mol
AGrx = what
Answers
Answer: \(\Delta G_{rxn}=130.19J\)
Explanation:
The balanced chemical reaction is,
\(CaCO_3(s)\rightarrow CaO(s)+CO_2(g)\)
The expression for Gibbs free energy change is,
\(\Delta G_{rxn}=\sum [n\times \Delta G_(product)]-\sum [n\times \Delta G_(reactant)]\)
\(\Delta G_{rxn}=[(n_{CO_2}\times \Delta G_{CO_2})+(n_{CaO}\times \Delta G_{CaO})]-[(n_{CaCO_3}\times \Delta G_{CaCO_3})]\)
where,
n = number of moles
Now put all the given values in this expression, we get
\(\Delta G_{rxn}=[(1\times -394.4)+(1\times -604.17)]-[(1\times -1128.76)]\)
\(\Delta G_{rxn}=130.19J\)
Therefore, the gibbs free energy for this reaction is, +130.19 kJ
Answer: 130 kJ
Explanation:
Science 5th grade very PLZ answer correctly TYSM WILL MARK AS BRAINLIST IF ANSWERED TODAY!


Answers
Answer:
A
Explanation:
Conducts electricity:
-Steel
-Gold
-Copper
-Aluminum
Does not conduct electricity:
-Plastic
-Glass
-Rubber
-Wood
Metallic bonding causes metals to conduct electricity. Some metals are more highly conductive than others.
Hope this helps :)
Answer:
The first table correctly classifies the materials.
Explanation:
In table 1, all the elements on the left side are transition metals, expect for steel. Steel is not an element, it's an alloy (Which is a combination of two or more elements).Transition metals are very good conductors of heat and electricity. The reason why transition metals make such good conductors is because their outer electrons can move freely in their atom.
The right side of table 1 correctly classifies the materials that do not conduct electricity. The reason why those materials are poor conductors is because they all have their electrons tightly bound to their atoms.
In table 2, it classifies copper has a poor conductor which is wrong. It also classifies wood as a good conductor which also wrong for the reasons I stated above.
Explain how the following reaction demonstrates that matter is neither created or destroyed in a chemical reaction: Ca(OH)2 + 2HCI-> CaCl2 + 2H20
Answers
Answer:
In this reaction, Ca(OH)2 is a reducing agent. It reacts with hydrogen chloride to form calcium chloride and water. Therefore, the following reaction shows that matter is neither created nor destroyed in a chemical reaction: Ca(OH)2 + 2HCI -> CaCl2 + 2H20. The formation of calcium chloride and water from the hydrolysis of calcium hydroxide is not an example of matter being created or destroyed in a chemical reaction because it does not involve the breaking down of any bonds between atoms.
Explanation:
A student decides to jump off a skateboard toward the east. Based on the action-reaction forces, which direction will the skateboard most likely move when she jumps off?
Answers
Answer:
l
Explanation:
Based on the action-reaction forces, the skateboard will most likely move towards west when she jumps off.
What is a force?Force is defined as a cause which is capable of changing the motion of an object. It can cause an object which has mass to change it's velocity. It is also simply a push or a pull . It has both magnitude as well as direction.Hence, it is a vector quantity.
It has SI units of Newton and is represented by'F'.Newton's second law states that force which acts on an object is equal to momentum which changes with time. If mass of object is constant, acceleration is directly proportional to net force acting on an object.
The concepts which related to force are thrust and torque .Thrust increases the velocity of an object and torque produces change in rotational speed of an object.
Learn more about force,here:
https://brainly.com/question/28875770
#SPJ6
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
Which of the following will have the highest melting point naphthalene C8H10 c. quartz SiO2 methane CH4 d. ethanol C2H5OH
Answers
Answer:
ethanol
Explanation:
the boiling and melting point directly proportional to the no. of polar hydroxyl group in the compound
Quartz has the highest melting point because it is a network solid.
The melting point of a solid gives us an idea of the kind of bonding that exists within the components solid.
The higher the melting point of the solid the stronger the interaction between the component particles that make up the solid.
Naphthalene, and methane are only held together by weak dispersion forces. The melting point of naphthalene is higher than that of methane because naphthalene has a higher molecular mass. Ethanol a lower melting point than naphthalene due to its low molecular mass irrespective of the presence of strong hydrogen bonding in the solid.
Quartz is a covalent network solid made up of SiO2 units. Covalent network solids are known to be strong and have high melting points because the sub-units are closely packed in the crystal.
Learn more: https://brainly.com/question/8236583

An iceberg has a volume of 0.1642 ML. What is the mass of the ice(in kg) composing the iceberg( at 0o C)? The density of ice at 0o C is 0.917g/cm^3
Answers
Answer:
1.5x10¯⁴Kg
Explanation:
Data obtained from the question include the following:
Volume = 0.1642mL = 0.1642cm³
Density = 0.917g/cm³
Mass =.?
The Density of a substance is simply defined as the mass per unit volume of the substance. Mathematically, it is represented as:
Density = Mass /volume
With the above formula, we can calculate the mass of the ice as follow:
0.917 = Mass / 0.1642
Cross multiply
Mass = 0.917 x 0.1642
Mass = 0.151g
Finally, we shall convert 0.1506g to kg. This is illustrated below:
1000g = 1k
Therefore, 0.151g = 0.151/1000 = 1.5x10¯⁴Kg
calculate the molar internal energy of carbon dioxide at 298.15k , taking it's translational and rotational degrees of freedom into consideration
Answers
Answer:
Explanation:
To calculate the molar internal energy of a gas at a given temperature, you need to know the molar specific heat capacities at constant volume and constant pressure for the gas. These values are typically provided in tables of thermodynamic data, which can be found in various sources such as textbooks or online. Since you mentioned that you want to take the translational and rotational degrees of freedom into consideration, you will need to use the molar specific heat capacity at constant volume, which accounts for these degrees of freedom.
Once you have the molar specific heat capacity at constant volume for the gas, you can use the equation U = Cv * T, where U is the molar internal energy, Cv is the molar specific heat capacity at constant volume, and T is the temperature in kelvins. In your case, the temperature is 298.15 K, so plugging in the appropriate values and solving for U will give you the molar internal energy of carbon dioxide at that temperature.
It's important to note that the molar specific heat capacity at constant volume is typically a function of temperature, so you will need to use the appropriate value for the temperature you are interested in. Additionally, different sources may provide slightly different values for the molar specific heat capacity, so it's always a good idea to consult multiple sources to get a sense of the range of possible values.
How many moles would be present in 300 liters of dinitrogen monoxide at
STP?
Answers
Answer: 13.4
Explanation: i guessed it and got it correct on ck-12
STP is the standard pressure and temperature condition of the ideal gas. 0.13 moles of dinitrogen monoxide will be present at STP.
What is the ideal gas equation?An ideal gas equation is a relation between the pressure, volume with moles, gas constant, and the temperature of the ideal gas. The ideal gas equation is given by,
\(\rm PV = nRT\)
Given at STP,
Pressure = 1 atm
Volume = 300 L
Moles = ?
Gas constant =8.3144598 J per mol per Kelvin
Temperature = 273 K
Substituting values in the ideal gas equation we get:
\(\begin{aligned} \rm n &= \rm \dfrac{ PV}{RT}\\\\&= \dfrac{1 \times 300}{273 \times 8.314}\\\\&=0.1321\end{aligned}\)
Therefore, 0.13 moles of dinitrogen monoxide are produced at STP.
Learn more about STP here:
https://brainly.com/question/15828565
The passage's author most vividly conveys the sense that Plumpp's poetry is like music when he
O uses words like "swing," "dance," and "sway" to characterize phrases in Plumpp's poems
O defines Plumpp as "the poet laureate of Chicago jazz and blues"
explains how long Plumpp has been writing about "Chicago jazz giants"
urges people to read Plumpp's poems and listen to the music Plumpp "immortalizes in print"
Answers
It is an amine, and it has less polar nitrogen-hydrogen and oxygen-hydrogen bonds.
A compound's boiling point is a physical characteristic. These intramolecular linkages between the molecules that make up a chemical affect these physical characteristics.
Alcohols and amino acids have the same kind of intermolecular linkages. The hydrogen bond is the name of this kind of bond.
The electrical attraction between a hydrogen atom from one molecule and an electronegative atom from a nearby molecule is known as a hydrogen bond.
The strength of the bond is in the following order: H.....F > H.....O > H......N
The H....N hydrogen bonds exist in amines, whereas the H....O hydrogen bonds exist in alcohols.
Consequently, the alcohol's hydrogen bonds are stronger and it will impart a higher boiling point on the compound.
Learn more about hydrogen bonds here-
https://brainly.com/question/10904296
#SPJ9
Answer:uses world like
Explanation:
how many moles are in 6.7 x 10^25 molecules of H2SO4
Answers
Answer:
\( \huge{ \boxed{111.30 \: \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L} \\ }\)
where
n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities.
From the question.
N = 6.7 × 10²⁵ \( \: H_2SO_4 \: \) molecules
\(n = \frac{6.7 \times {10}^{25} }{6.02 \times {10}^{23} } \\ = 111.2956...\)
We have the final answer as.
111.30 molesGiven this equation: 2 H2 + O2 → 2 H2O, how many moles of O2 are needed to yield 0.3 moles of water?
Answers
From the euqation, we can see that 1 mol of O₂ reacts to produce 2 moles of H₂O.
We can use this and rule of three to calculate the answer:
1 mol O₂ --- 2 mol H₂O
x --- 0.3 mol H₂O
So:
\(\begin{gathered} \frac{1}{x}=\frac{2}{0.3} \\ \frac{x}{1}=\frac{0.3}{2} \\ x=\frac{0.3}{2}=0.15 \end{gathered}\)So, we need 0.15 mol of O₂.
What happens when an acid reacts with a metal such as sodium?
The temperature decreases.
The acid is converted to a base.
A chemical reaction occurs.
The metal becomes polished and shiny.
Answers
Answer:
a chemical reaction occurs
Predict and explain the structure of the major and minor products when hydrogen bromide is added to 2-methylbut-2- ene, (Ch3)2CCHCH3
Pls help with homework!!!!
Answers
When hydrogen bromide (HBr) is added to 2-methylbut-2-ene ((CH3)2CCHCH3), an electrophilic addition reaction takes place, where the π bond of the alkene is broken, and the hydrogen and bromine atoms are added to the resulting carbocation.
The reaction proceeds through a Markovnikov addition, where the hydrogen atom attaches to the carbon atom with the greater number of hydrogen atoms.
In this case, the initial addition of HBr to 2-methylbut-2-ene leads to the formation of a primary carbocation, as the positively charged carbon atom only has one alkyl group attached to it. The primary carbocation is relatively unstable, and it can undergo a rearrangement to form a more stable secondary carbocation.
The major product that is typically obtained is the 2-bromo-2-methylbutane. The hydrogen atom from HBr adds to the carbon with three hydrogen atoms (the more substituted carbon), resulting in the formation of a secondary carbocation.
On the other hand, a minor product is also formed, which is 3-bromo-2-methylbutane. This product arises from the addition of HBr to the primary carbocation, which is less stable. Although the primary carbocation is less favored, it can still be formed and lead to the formation of the minor product.
In summary, the addition of HBr to 2-methylbut-2-ene yields two products: the major product is 2-bromo-2-methylbutane, resulting from the addition of HBr to the more stable secondary carbocation, and the minor product is 3-bromo-2-methylbutane, originating from the less stable primary carbocation.
For more such questions on electrophilic addition visit:
https://brainly.com/question/9643304
#SPJ8
How can a good or service affect culture?
Answers
Answer:
Explanation:
establish a direct relationship between organizational culture and effectiveness
under what circumstances do you think credit cards should NOT be used ?
Answers
It's never a good idea to use your credit card when experiencing strong emotions, especially if you tend to steer toward 'retail therapy.
How has the U.S. Census Bureau changed the way it records information about relationships?
A. leaving descriptions of relationship type open-ended to allow the respondent to describe them
B. increasing the number of relationship categories to include same-sex partner options
B. ceasing to ask about relationship status altogether and only asking about cohabitation
D. ceasing to ask about marriage and using only "partner" designations
Answers
U.S. Census Bureau changed the way it records information about relationships by increasing the number of relationship categories to include same-sex partner options and is denoted as option B.
What is Relationship?This is a close connection between people and is characterized by physical or emotional intimacy.
There has been changes in the way U.S. Census Bureau records information is recorded in this aspect by increasing the number of relationship categories for inclusiveness of other types of people such as those who have same-sex partners and so on.
This is therefore the reason why option B was chosen as the correct choice.
Read more about Relationship here https://brainly.com/question/10286547
#SPJ1
