A solution contains 0.600 g Mg2 in enough water to make a 1875 mL solution. What is the milliequivalents of Mg2 per liter (mEq/L) for this solution
Answers
The milliequivalents of Mg2 per liter (mEq/L) for this solution is 3.2 mEq/L or 0.013 mEq/L.
To calculate the milliequivalents of Mg2 per liter (mEq/L), we need to know the molar mass of Mg2, which is the formula weight of Mg2.
The formula weight of Mg2 is calculated as follows:
Formula weight of Mg2 = 2 × atomic weight of Mg
The atomic weight of Mg is approximately 24.31 g/mol.
Therefore:
Formula weight of Mg2 = 2 × 24.31 g/mol
Formula weight of Mg2 = 48.62 g/mol
Now, we need to determine the number of moles of Mg2 present in the solution. To do this, we divide the mass of Mg2 in grams by its molar mass:
Number of moles of Mg2 = Mass of Mg2 / Formula weight of Mg2
Given that the mass of Mg2 in the solution is 0.600 g, we can calculate the number of moles:
Number of moles of Mg2 = 0.600 g / 48.62 g/mol
Number of moles of Mg2 ≈ 0.01234 mol
Next, we need to calculate the milliequivalents (mEq) of Mg2. Since Mg2 carries a double positive charge, the number of milliequivalents is twice the number of moles:
Milliequivalents of Mg2 = 2 × Number of moles of Mg2
Milliequivalents of Mg2 = 2 × 0.01234 mol
Milliequivalents of Mg2= 0.02468 mEq
Finally, we need to determine the mEq/L concentration. We know that the solution has a volume of 1875 mL, which is equivalent to 1.875 L:
mEq/L concentration = Milliequivalents of Mg2 / Volume of solution in liters
mEq/L concentration = 0.02468 mEq / 1.875 L
mEq/L concentration ≈ 0.01314 mEq/L
Rounding to the appropriate number of significant figures, the milliequivalents of Mg2 per liter (mEq/L) for this solution is approximately 0.013 mEq/L or 3.2 mEq/L.
The milliequivalents of Mg2 per liter (mEq/L) for the given solution is 3.2 mEq/L.
To know more about solution visit:
https://brainly.com/question/25326161
#SPJ11
Related Questions
An automobile gasoline tank holds 25.0 gal when full. how many pounds of gasoline will it hold if the gasoline has a density of 0.737 g/ml ?
Answers
The amount (in pounds) the gasoline tank will hold when full is 153.76 lb
What is density?The density of a substance is simply defined as the mass of the subtance per unit volume of the substance. Mathematically, it can be expressed as
Density = mass / volume
How to convert gallon to milliliters1 gallon = 3785.412 mL
Therefore,
25 gallon = 25 × 3785.412
25 gallon = 94635.3 mL
How to determine the mass Density = 0.737 g/mLVolume = 94635.3 mLMass =?Mass = Density × Volume
Mass = 0.737 × 94635.3
Mass = 69746.2161 g
How to convert grams to pounds453.592 g = 1 lb
Therefore,
69746.2161 g = 69746.2161 / 453.592
69746.2161 g = 153.76 lb
Learn more about density:
https://brainly.com/question/952755
#SPJ1
This type of intermolecular force relates to the
big difference in the electronegativities. It exists
when N, O or F atom attracts with hydrogen
atom. Medium strength of attraction is
exhibited
Answers
The type of intermolecular force relates to the big difference in the electronegativities and is known as hydrogen bonding.
What are intermolecular forces?Intermolecular forces may be defined as the mechanism that mediates the interaction between the atoms of the molecules. It significantly includes the electromagnetic forces of attraction and repulsion between the atoms.
Some examples of intermolecular forces include London-dispersion force, dipole-dipole interaction, van der Waals interaction, hydrogen bonds, etc. Each one of them possesses a different set of characteristic properties distinctly. Some types of forces are also present in the body of living organisms.
Hydrogen bonding is a characteristic kind of interaction that remarkably includes dipole-dipole attraction between the most electronegative elements like Nitrogen, Oxygen, fluorine, etc., and the hydrogen atom.
Therefore, hydrogen bonding is a type of intermolecular force relates to the big difference in the electronegativities.
To learn more about Intermolecular forces, refer to the link:
https://brainly.com/question/13588164
#SPJ2
is.
O2(g) + 2H2O(l) + 4e- -----------------> 4OH−(aq)
oxidation or reduction?
Answers
Answer:
Reduction
Explanation:
Oxidation is a process that involves loss of electrons.
Reduction is a process that involves gain of electrons.
In this equation, the electrons (4e-) are in the reactant side. This means that it is gain of electrons.
The reaction is a reduction reaction.
How does the law of multiple proportions support the idea of the atom?
Answers
According to the research, the correct answer is that the law of multiple proportions applies to different compounds made up of the same elements where the atoms were indivisible and two or more elements could chemically join together to form compounds.
What is the Law of multiple proportions?It is a law was formulated by the British chemist John Dalton in 1803 and states that when the combination of elements exists in more than one proportion to form different compounds, the ratio between the masses of one of the elements is expressed in small integers.
In this sense, it explains that a pure element consists of indivisible particles called atoms that are all the same within the element and can be identified by their atomic weight.
Therefore, we can conclude that according to the research, the correct answer is that the law of multiple proportions applies to different compounds made up of the same elements where the atoms were indivisible and two or more elements could chemically join together to form compounds.
Learn more about the law of multiple proportions here: https://brainly.com/question/28008352
#SPJ1
Which fraction contains the largest molecules?
A) diesel
B)gasoline
C)kerosene?
Answers
Answer the following questions. Explain your answer further.
1. Is the hydrostatic pressure the same along any constant horizontal line?
2. What is the effect of temperature on the hydrostatic pressure?
Answers
1. The hydrostatic pressure varies along different horizontal lines due to differences in depth.
2. The temperature, although capable of affecting the density of the fluid, does not influence hydrostatic pressure.
The hydrostatic pressure is not the same along any constant horizontal line. The pressure at a specific point depends on its depth within the fluid. If two points have the same depth, they will experience the same pressure. However, if two points are located at different horizontal lines but have the same depth, their pressures will also be equal. On the other hand, when the depths of two points differ, the pressure at the deeper point will be greater than that at the shallower point.
In terms of temperature, its direct effect on hydrostatic pressure is negligible. Hydrostatic pressure is primarily determined by the depth of the point and the density of the fluid. Assuming a constant fluid density, the temperature has no immediate impact on hydrostatic pressure. However, the temperature can indirectly influence pressure by altering the fluid's density. When the temperature of the fluid increases, its density decreases. Consequently, this reduction in density leads to a decrease in hydrostatic pressure since pressure is directly proportional to fluid density. Nevertheless, the change in hydrostatic pressure resulting from temperature fluctuations is generally small, and it can usually be disregarded in most practical applications.
In summary, the hydrostatic pressure varies along different horizontal lines due to differences in depth. The temperature, although capable of affecting the density of the fluid, has only a minor influence on hydrostatic pressure and is typically not a significant consideration in practical scenarios.
Learn more about hydrostatic pressure from the link given below:
brainly.com/question/25715580
#SPJ11
The half-life of Radon-222 is 3.8 days. If a 10 gram sample is present, how many days will it take to have less than one gram remaining?
Answers
(alt + F4) might be the answer you are looking
Explanation:
I'm not exactly sure how I came up with this answer but it is for sure the answer
The other station has a solution of sodium bicarbonate (formula: nahco₃) and citric acid (formula: hoc(co2h)(ch2co2h)2).
na2hco3 (aq) + h3c3h5o7(aq) → na3c3h5o7(aq) + h2co3 (aq)
type of reaction? ___________________________________
the carbonic acid produced in this reaction keeps reacting to produce water and carbon dioxide
h2co3 (aq) → h2o(l) + co2(g)
type of reaction? decomposition
iii. notice the symbols inside the parentheses after the formula of the compounds. what do they mean?
s
l
g
aq
Answers
The type of reaction for the given equation is a double displacement reaction, where the sodium bicarbonate and citric acid react to form sodium citrate and carbonic acid. The carbonic acid then undergoes a decomposition reaction to produce water and carbon dioxide. This type of reaction is called a decomposition reaction.
The symbols inside the parentheses after the formula of the compounds represent the chemical structure of the molecule. In the case of citric acid, the parentheses indicate the presence of three carboxylic acid functional groups, which are responsible for its acidity.
The presence of these groups also allows for the reaction with sodium bicarbonate to occur, forming sodium citrate and carbonic acid. Overall, this reaction demonstrates the principles of acid-base chemistry and the importance of understanding chemical structures in predicting reactions.
To know more about given equation is a double displacement refer here
https://brainly.com/question/14057630#
#SPJ11
Calculate the number of molecules in 12.5 mol of CaCO3
Answers
The number of molecules in 12.5 moles of CaCO₃ is 7.525×10²⁴ molecules
How do I determine the number of molecules?From Avogadro's hypothesis, we understood that
1 mole of CaCO₃ = 6.02×10²³ molecules
Using the above information, we can obtain the number of molecules in 12.5 moles of CaCO₃ as illustrated below:
From Avogadro's hypothesis,
1 mole of CaCO₃ = 6.02×10²³ molecules
Therefore,
12.5 moles of CaCO₃ = (12.5 moles × 6.02×10²³ molecules) / 1 mole
12.5 moles of CaCO₃ = 7.525×10²⁴ molecules
Thus, we can conclude from the calculation made above that the number of molecules is 7.525×10²⁴ molecules
Learn more number of molecules:
https://brainly.com/question/20572558
#SPJ1
The best Lewis structure of HNO has [ Select ] on the central atom. How many electron groups are around the central atom in HNO
Answers
The best Lewis structure of HNO has a double bond on the central atom. There are 3 electron groups around the central atom in HNO.
The electronic configuration of Hydrogen is 1s¹, and that of nitrogen is 1s²2s²2p³. Nitrogen has five valence electrons and hydrogen has one valence electron. The total number of valence electrons in HNO can be calculated by summing the valence electrons of each atom. This gives:1(H) + 5(N) + 6(O) = 12 valence electrons .
How many electron groups are around the central atom in HNO?
There are three electron groups around the central atom of nitrogen in HNO. Two of these electron groups are lone pairs and one is a bond pair. The bond pair is made up of a nitrogen-oxygen double bond. The Lewis structure of HNO is as follows: Therefore, the best Lewis structure of HNO has a double bond on the central atom and there are 3 electron groups around the central atom in HNO.
To learn more about Lewis structure visit below link
https://brainly.com/question/29603042
#SPJ11
2c2h2(g) + 5o2(g) → 4co2(g) + 2h2o(g) this is a balanced equation for the combustion of acetylene (c2h2). how many grams of acetylene(c2h2 ) are required to produce 1.0 moles of co2?
Answers
13.015 grams of acetylene (C2H2) are required to produce 1.0 moles of CO2.
To find the number of grams of acetylene (C2H2) required to produce 1.0 moles of CO2, we can use the balanced equation for the combustion of acetylene: 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g)
From the balanced equation, we know that for every 2 moles of C2H2 that react, 4 moles of CO2 are produced. Therefore, if we want to produce 1.0 moles of CO2, we need to react 1.0 moles of CO2 / 4 moles CO2/ 2 moles C2H2 = 0.5 moles of C2H2.
To convert moles to grams, we use the molar mass of C2H2 which is 26.03 g/mol
So, 0.5 moles of C2H2 = 0.5 x 26.03 g/mol = 13.015 g C2H2
Therefore 13.015 grams of acetylene (C2H2) are required to produce 1.0 moles of CO2
For more questions like Acetylene click the link below:
https://brainly.com/question/26432975
#SPJ4
which compound is likely to conduct electricity when in aqueous solution? 1. phosphorous trichloride 2. carbon dioxide 3. calcium chloride 4. none of these is likely to conduct electricity in aqueous solution. 5. sulfur trioxide
Answers
The compound is likely to conduct electricity when in an aqueous solution of calcium chloride. Option 3.
Sodium chloride in its aqueous state can conduct electricity due to the presence of free ions, whereas urea glucose and sucrose, which are not electrolytes, do not conduct electricity. An aqueous solution is a water that contains one or more solutes.
Solutes in aqueous solutions can be solids gases or other liquids. A mixture must be stable to be a true solution. Aqueous in a chemical formula indicates that the substance is dispersed in water. For example, if you pour caustic soda into dilute hydrochloric acid, you get an aqueous solution of sodium chloride. The liquid is used to indicate the formation of liquid substances in reactions.
Learn more about Sodium chloride here:-https://brainly.com/question/24878544
#SPJ4
Calculate the number of grams Fe2O3 produced from 8.93 g 02.
4 Fe +302 → 2 Fe2O3
Answers
Hey there!:
Molar mass :
Fe₂O₃ = 159.69 g/mol
O₂ = 31.99 g/mol
By reaction stochiometry :
4 Fe + 3 O₂ = 2 Fe₂O₃
3 * (31.99 g O₂ ) ------------------ 2 * ( 159.69 ) g Fe₂O₃
8.93 g O₂ --------------------------- ( mass O₂)
mass O₂ = ( 8.93 * 2 * 159.69 ) / ( 3 * 31.99 )
mass O₂ = 2852.0634 / 95.97
mass O₂ = 29.71 g Of Fe₂O₃
Hope this helps!
The ornithine decarboxylase reaction has been studied extensively by biomedical researchers. the most likely reason for the interest of these researchers is that the reaction is:_________.
Answers
The ornithine decarboxylase reaction has been studied extensively by biomedical researchers the most likely reason for the interest of these researchers is that the reaction is an early event in the cell division
Ornithine decarboxylase
The enzyme ornithine decarboxylase catalyzes the decarboxylation of ornithine to form putrescine. This reaction is the committed step in polyamine synthesis.Transmission of genetic information from the gene to the protein because the passage states that in mammals and other organisms ornithine decarboxylase participates in early events in cell division.
Role of the ornithine decarboxylase
Ornithine decarboxylase is an important enzyme involved in the polyamine biosynthesis of all living cells. This enzyme causes decarboxylation of ornithine to form putrescine, which is subsequently converted into spermidine and spermine.Hence the ornithine decarboxylase reaction has been studied extensively by biomedical researchers the most likely reason for the interest of these researchers is that the reaction is an early event in the cell division.
Learn more about the chemical reaction on
https://brainly.com/question/16416932
#SPJ4
Identify the key differences between the Articles of Confederation and the U.S. Constitution. Then explain which document created the better system of government for the new nation, and support your response with the differences you have identified.
22 POINTS + WILL RATE BRAINLEST!!!!!
Answers
The key differences between the Articles of Confederation and the U.S Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
The document created the better system of government for the new nation is the US constitution.
The Article of confederation , the state have stronger power than the central power and in the US constitution , the power of central government is stronger than the states. The important development was the establishment of three departments. There are three departments of government that is legislative , executive and judicial.
Thus, The key differences between the Articles of Confederation and the U.S. Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
To learn more about US Constitution here
https://brainly.com/question/29027032
#SPJ1
explain carbon movement into the air during cellular respiration.
Answers
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this
information, what can Derek infer about horses and donkeys?
Horses and donkeys cannot survive in the same
environment.
Horses and donkeys produce fertile offspring.
Horses and donkeys are members of the same
population.
Horses and donkeys are members of different
populations.
Answers
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this information, Derek can infer that horses and donkeys are members of different populations.
Since horses and donkeys are different species, they belong to different populations. A population refers to a group of individuals of the same species that live in the same area and can interbreed. While horses and donkeys can mate, their offspring, known as mules, are usually infertile.
This means that mules cannot produce offspring of their own, which indicates that horses and donkeys are not members of the same population. In contrast, if they were members of the same population, they would be able to produce fertile offspring. Therefore, Derek can infer that horses and donkeys are members of different populations.
Learn more about mules here:
https://brainly.com/question/15852289
#SPJ11
Provide 4 examples of each of the following, what are they used for and their environmental health and safety impacts: - Natural Nanomaterial - Engineered Nano materials - Organic Nano materials - Inorganic Nanomaterials
Answers
Nanomaterials, whether natural, engineered, organic, or inorganic, offer various applications across industries. However, their environmental health and safety impacts need to be carefully evaluated and managed to mitigate any potential risks.
Understanding their properties, fate, and behavior in different environments is crucial for responsible development, use, and disposal of nanomaterials.
Natural Nanomaterials:
Examples: Carbon nanotubes (CNTs) derived from natural sources like bamboo or cotton, silver nanoparticles in natural colloids, clay minerals (e.g., montmorillonite), iron oxide nanoparticles found in magnetite.
Uses: Natural nanomaterials have various applications in medicine, electronics, water treatment, energy storage, and environmental remediation.
Environmental health and safety impacts: The environmental impacts of natural nanomaterials can vary depending on their specific properties and applications. Concerns may arise regarding their potential toxicity, persistence in the environment, and possible accumulation in organisms. Proper disposal and regulation of their use are essential to minimize any adverse effects.
Engineered Nanomaterials:
Examples: Gold nanoparticles, quantum dots, titanium dioxide nanoparticles, carbon nanomaterials (e.g., graphene), silica nanoparticles.
Uses: Engineered nanomaterials have widespread applications in electronics, cosmetics, catalysis, energy storage, drug delivery systems, and sensors.
Environmental health and safety impacts: Engineered nanomaterials may pose potential risks to human health and the environment. Their small size and unique properties can lead to increased toxicity, bioaccumulation, and potential ecological disruptions. Safe handling, proper waste management, and risk assessment are necessary to mitigate any adverse effects.
Organic Nanomaterials:
Examples: Nanocellulose, dendrimers, liposomes, organic nanoparticles (e.g., polymeric nanoparticles), nanotubes made of organic polymers.
Uses: Organic nanomaterials find applications in drug delivery, tissue engineering, electronics, flexible displays, sensors, and optoelectronics.
Environmental health and safety impacts: The environmental impact of organic nanomaterials is still under investigation. Depending on their composition and properties, they may exhibit varying levels of biocompatibility and potential toxicity. Assessments of their environmental fate, exposure routes, and potential hazards are crucial for ensuring their safe use and minimizing any adverse effects.
Inorganic Nanomaterials:
Examples: Quantum dots (e.g., cadmium selenide), metal oxide nanoparticles (e.g., titanium dioxide), silver nanoparticles, magnetic nanoparticles (e.g., iron oxide), nanoscale zeolites.
Uses: Inorganic nanomaterials are utilized in electronics, catalysis, solar cells, water treatment, imaging, and antimicrobial applications.
Environmental health and safety impacts: Inorganic nanomaterials may have environmental impacts related to their potential toxicity, persistence, and release into ecosystems. Their interactions with living organisms and ecosystems require careful assessment to ensure their safe use and minimize any negative effects.
Understanding their properties, fate, and behavior in different environments is crucial for responsible development, use, and disposal of nanomaterials.
To know more about Nanomaterials, visit
brainly.com/question/29540028
#SPJ11
Phosphoric acid, H3PO4(aq),H3PO4(aq), is a triprotic acid, meaning that one molecule of the acid has three acidic protons. Estimate the pH and the concentrations of all species in a 0.350 M phosphoric acid solution.
Answers
The estimated pH of the 0.350 M phosphoric acid solution is approximately 1.0, and the concentrations of all species in the solution are approximately:
[H₃PO₄] ≈ 0.350 M
[H₂PO₄⁻] ≈ 0.1 M
[HPO₄²⁻] ≈ 0.1 M
[PO₄³⁻] ≈ 0.1 M
[H⁺] ≈ 0.1 M
pH is a measure of the acidity or alkalinity of a solution. It is defined as the negative logarithm (base 10) of the concentration of hydrogen ions ([H+]) in the solution. Mathematically, the pH is calculated as:
pH = -log10[H+]
The pH scale ranges from 0 to 14, where pH 7 is considered neutral. Solutions with a pH less than 7 are acidic, and solutions with a pH greater than 7 are alkaline or basic. The lower the pH value, the more acidic the solution, while higher pH values indicate greater alkalinity.
To estimate the pH and the concentrations of all species in a 0.350 M phosphoric acid (H₃PO4) solution, we need to consider the ionization reactions of each acidic proton.
Phosphoric acid, H₃PO₄, ionizes in water as follows:
1st ionization: H₃PO₄ ⇌ H⁺+ H₂PO₄⁻
2nd ionization:H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻
3rd ionization: HPO₄²⁻⇌ H⁺ + PO₄³⁻
Let's denote the initial concentration of phosphoric acid as [H₃PO₄]⁻initial = 0.350 M. Since it is a triprotic acid, we'll have three ionization steps.
1st ionization:
[H₃PO₄]⁻initial = [H⁺] + [H₂PO₄⁻]
Since the concentration of [H₃PO₄]⁻ initially is much greater than the concentrations of the other species initially, we can assume that x, the concentration of H⁺ and [H₂PO₄⁻], will be negligible compared to 0.350 M. Thus, we can approximate [H₃PO₄]⁻initial ≈[H₂PO₄⁻].
2nd ionization:
[H₂PO₄⁻] ≈ [H⁺] + [HPO₄²⁻]
Again, assuming x is negligible compared to 0.350 M, we can approximate [H₂PO₄⁻] ≈ [HPO₄²⁻].
3rd ionization:
[HPO₄²⁻] ≈ [H⁺] + [PO₄³⁻]
Once more, assuming x is negligible compared to 0.350 M, we can approximate [HPO₄²⁻] ≈ [PO₄³⁻].
Using the approximation, we can set up an equilibrium table:
| [H₃PO₄] | [H₂PO₄⁻] | [HPO₄²⁻] | [PO₄³⁻] | [H⁺]
Initial | 0.350 M | 0 M | 0 M | 0 M | 0 M
Change | -x | +x | +x | +x | +x
Equilibrium | 0.350-x | x | x | x | x
Since the ionization constant (Ka) for each step is relatively small, we can assume that x is small compared to the initial concentration of phosphoric acid (0.350 M).
1st ionization: Ka1 = ([H⁺][H₂PO₄⁻])/[H₃PO₄] ≈ x²/(0.350)
2nd ionization: Ka2 = ([H⁺][HPO₄²⁻])/[H2PO₄⁻] ≈ x²/x ≈ x
3rd ionization: Ka3 = ([H⁺][PO₄³⁻])/[HPO₄²⁻] ≈ x²/x ≈ x
We can solve for x by taking the square root of Ka1 and using that value to calculate Ka2 and Ka3:
x = sqrt(Ka1) ≈ sqrt(1.0 x 10⁻²)
Using this value of x, we can calculate the concentrations:
[H+] ≈ x ≈ sqrt(1.0 x 10⁻²) ≈ 0.1 M
[H₂PO₄⁻] ≈ [HPO₄²⁻] ≈ [PO₄³⁻] ≈ x ≈ 0.1 M
The pH of the solution is given by the negative logarithm of the hydrogen ion concentration:
pH ≈ -log10(0.1) ≈ 1.0
To know more about pH visit:
https://brainly.com/question/26856926
#SPJ11
Which body systems help to exchange oxygen and carbon dioxide?
A. respiratory and digestive
B. circulatory and digestive
C. respiratory and circulatory
D. digestive and excretory
Answers
calculate the ph at equivalence point and find the indicators for the following titrations:
a. 25 mL of 0.5 M HI with 0.1 M KOH b. 25 mL 0.1 M CH3NH2 with 0.1 M HCI c. 25 mL of 0.1 M CH3COOH with 0.1 M HCI
Answers
The pH at the equivalence point for the titration of 25 mL of 0.5 M HI with 0.1 M KOH is approximately 7.
In this titration, HI is a strong acid, and KOH is a strong base. At the equivalence point, the moles of acid are equal to the moles of base, resulting in the formation of water and a salt. The salt formed in this reaction is KI. KI is a neutral salt, so the pH at the equivalence point is approximately 7, which is neutral.
Since the salt formed at the equivalence point is neutral, the pH is 7. Therefore, no indicator is required for this titration.
To know more about pH follow the link:
https://brainly.com/question/172153
#SPJ11
For the reaction: 2H₂+O₂ -> 2H₂O, how many grams of water are produced from 6.00 moles of H₂?
Answers
The number of grams of water that are produced from the moles of H₂ is 108.09 grams .
How to find the number of grams produced ?From the balanced chemical equation, we see that 2 moles of H₂ reacts to produce 2 moles of H₂O. Therefore, 1 mole of H₂ reacts to produce 1 mole of H₂O.
To find the number of moles of water produced from 6.00 moles of H₂, we can use the stoichiometry of the balanced chemical equation:
6.00 moles H₂ x (2 moles H₂O / 2 moles H₂) = 6.00 moles H₂O
So 6.00 moles of H₂ produces 6.00 moles of H₂O. To convert moles of water to grams, we need to use the molar mass of water:
Molar mass of H₂O = 2(1.008 g/mol) + 1(15.999 g/mol) = 18.015 g/mol
So, the mass of 6.00 moles of H₂O is:
6.00 moles H₂O x 18.015 g/mol = 108.09 g
Find out more on grams produced at https://brainly.com/question/20703641
#SPJ1
A material that lets electricity pass through it is called ...
Answers
Answer:
Conductors
Explanation:
Hope this helps!
HELP CHEM ASAP PLZZZ!!!! Brainlist

Answers
Answer:
True.
Explanation:
The higher the 'Mol Concentration', the stronger the acid or base the substance is. For example...
1 Mol of HCl is less concentrated than 6 Mol HCl.
This is the same with bases:
1 Mol of NaOH is less concentrated than 6 Mol NaOH.
Someone plz help I Don’t know I would do something like this and I really need to get it done
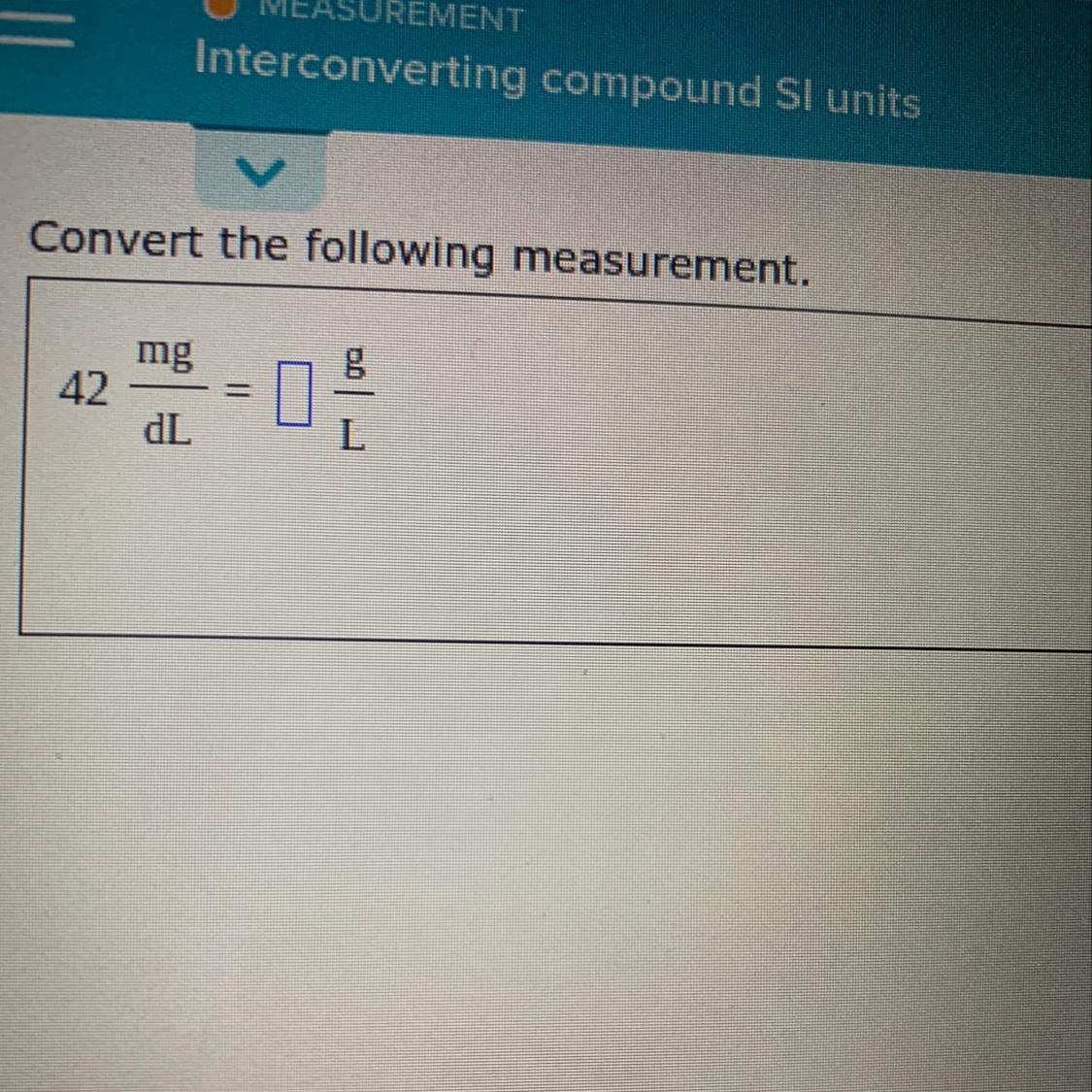
Answers
1dL =.1L
0.001/.1 *42=0.42
A central atom has two lone pairs on opposite sides and four single bonds. What is the molecule geometry of the result?
Answers
The molecular geometry is square planar.
According to the Valence Shell Electron Pair Repulsion theory (VSEPR), the shape of a molecule is determined by the number of valence electrons surrounding the outermost shell of the central atom in the molecule.
In this case, the expected geometry based on VSEPR theory is octahedral. However, the lone pairs on opposite sides of the four single bonds leads to a square planar molecular geometry.
Learn more; https://brainly.com/question/24396703
what is the difference between quantative and qualitative analysis
Answers
Answer:
I guess quantitative data is countable or measurable, relating to numbers; qualitative data is descriptive, relating to words. Quantitative data lends itself to statistical analysis; qualitative data is grouped and categorized according to themes.
Explanation:
Answer:
quantitative - actual numbers as data
Qualitative- uses senses (taste, touch, etc.)
Explanation:
A poison that prevents the transfer of electrons from the last [Fe-S] cluster of Complex I to coenzyme Q is added to a suspension of actively respiring mitochondria. Which of the following will be observed? a) ATP production would be impaired due to the uncoupling of oxidative phosphorylation from electron transport. b) ATP production would be reduced due to inhibition of the CoQ subunit of ATP synthase. c) ATP production would be reduced due to a decrease in the number of protons pumped out of the mitochondrial matrix. d) ATP production would be halted completely due to the block in electron transport through the electron transport chain.
Answers
When a poison that prevents the transfer of electrons from the last [Fe-S] cluster of Complex I to coenzyme Q is added to a suspension of actively respiring mitochondria, the observed ATP production would be halted completely due to the block in electron transport through the electron transport chain.
Oxidative phosphorylation is the process in which ATP is produced by the transfer of electrons in a mitochondrion from an electron donor to an electron acceptor with oxygen as the terminal acceptor.
This is coupled with the generation of a proton gradient across the inner mitochondrial membrane by a series of electron carriers.
The poisoning of the transfer of electrons from the last [Fe-S] cluster of Complex I to coenzyme Q leads to the blocking of the transfer of electrons down the electron transport chain from NADH to molecular oxygen, and the production of ATP is stopped.
This will be observed in the observed ATP production would be halted completely due to the block in electron transport through the electron transport chain.
Therefore, ATP production would be halted completely due to the block in electron transport through the electron transport chain.
For more information on ATP electrons refer https://brainly.com/question/25532497
#SPJ11
calculate the molarity of a 10.0% cacl₂ solution. the density of the solution is 1.0835 g/cm³.
Answers
The molarity of the 10.0% CaCl₂ solution is 0.9007 M
Molarity of a solution is defined as the number of moles of solute present in 1 litre of the solution.
To calculate the molarity of the CaCl₂ solution, we need to first determine the mass of CaCl₂ present in one liter i.e. 1000 ml of the solution.
A 10.0% CaCl2 solution means that 10.0 g of CaCl₂ is present in 100 g of the solution.
We can use this information to calculate the mass of CaCl2 in one liter of the solution as follows:
Mass of CaCl₂ in 1 L
= (10.0 g CaCl₂ / 100 g solution) x (1000 g solution / 1 L solution)
∴ Mass of CaCl₂ in 1 L = 100 g CaCl₂ / 1 L solution
Now since we know the mass of CaCl₂ in one liter of the solution, we can use the molarity formula to calculate the molarity of the solution:
Molarity = moles of solute / volume of solution (in liters)
To find the moles of CaCl₂ present in one liter of the solution, we need to divide the mass of CaCl₂ by its molar mass.
The molar mass of CaCl₂ is 110.98 g/mol
moles of CaCl₂ in 1 L
= 100 g CaCl₂ / 110.98 g/mol
= 0.9007 mol
Finally, we can use the formula for molarity to calculate the molarity of the solution:
Molarity = moles of solute / volume of solution in L
Molarity
= 0.9007 mol / 1 L
= 0.9007 M
Thus, molarity of the solution is 0.9007 M
To know more about molarity here
https://brainly.com/question/30792756
#SPJ4
Microscopic interface asymmetry and spin-splitting of electron subbands in semiconductor quantum structures. Solid State Commun
Answers
The microscopic interface asymmetry of grown semiconductor heterostructures.
The dispersion of restricted electrons. beginning from a multiband envelope formulation we practice matrix perturbation theory to derive specific expressions. Interface asymmetry, which in the conduction band Hamiltonian appear as a warping and a spin-splitting term. The warping term consequences in an inequivalence of the dispersion.
The microscopic interface asymmetry of grown semiconductor heterostructures that gives upward thrust to heavy-light hole coupling even at 0 in-plane wave vector, modifies also the dispersion of restricted electrons. beginning from a multiband envelope method we practice matrix perturbation principle to derive explicit expressions as a result of this interface asymmetry, which inside the conduction band.
Learn more about Microscopic interface here:-https://brainly.com/question/26348652
#SPJ4