Answers
Answer:
Yes, you need right amount of both though
Explanation:
Related Questions
Look at the reaction below. Upper H subscript 2 upper S upper O subscript 4 (a q) plus upper Upper M g (s) right arrow Uper M g upper S upper O subscript 4 (a q) plus upper H subscript 2 (g). Which substance is the acid in the reaction? Mg(s) H2(g) MgSO4(aq) H2SO4(aq)
Answers
Answer:
H2SO4(aq)
Explanation:
The balanced equation for the reaction is given below:
H2SO4(aq) + Mg(s) —> MgSO4(aq) + H2 (g)
An acid is a substance which dissolves in water to produce hydrogen ion, H+ as the only positive ion.
To know which of the substance is acid, let us dissolve them in water to see which will produce hydrogen ion, H+ as the only positive ion.
This is illustrated below:
H2SO4(aq) —> 2H+(aq) + SO4^2-(aq)
Mg(s) + 2H2O(l) —> Mg(OH)2(aq) + H2(g)
MgSO4(aq) —> Mg^2+(aq) + SO4^2-(aq)
H2 is insoluble in water.
From the above, only H2SO4 produces hydrogen ion H+ on dissolution in water. Therefore, H2SO4 is an acid
Answer:
D on edg 2021
Explanation:
In the reaction H₂CO3 + 2KOH --> K₂CO3 + 2H₂O the salt is...
Answers
Answer:
K2CO3 is the salt
Explanation:
K2CO3 is the salt
since, H2O is a liquid
so, it's lefting K2CO3 a salt.
If 450 grams of dextrose are dissolved in 650ml Of water with a resultant volume of 1 liter what is the percent concentration (w/v) of dextrose
Answers
Considering the definition of mass volume percentage, the percent concentration of dextrose is 45%.
Mass volume percentageMass volume percentage (% m/V) is a measure of concentration that indicates the number of grams of solute per 100 volume units of the solution. In other words, the volume percent of a component in the solution is defined as the ratio of the mass of the component to the volume of the solution, expressed as a percentage.
The mass volume percentage of a solution is determined by the following expression:
\(Mass volume percentage=\frac{mass of solute}{volume of solution}x100\)
Percent concentration in this caseIn this case, you know:
mass of solute= 450 gramsvolume of solution= 1 L= 1000 mLReplacing in the definition of mass volume percentage:
\(Mass volume percentage=\frac{450 grams}{1000 mL}x100\)
Solving:
mass volume percentage= 45%
Finally, the percent concentration of dextrose is 45%.
Learn more about mass volume percentage:
https://brainly.com/question/14471247
https://brainly.com/question/18850112
#SPJ1
A bucket contains 15.0 L of seawater. The density of seawater is 1.027 g/mL.
What is the mass of the seawater?
Report your answer using the correct amount of significant figures and the appropriate unit.
Answers
\(\\ \sf\longmapsto Density=\dfrac{Mass}{Volume}\)
\(\\ \sf\longmapsto Mass=Density\times Volume\)
\(\\ \sf\longmapsto Mass=0.1027(15)\)
\(\\ \sf\longmapsto Mass=0.1925kg\)
The mass unit associated with density is usually grams. If the volume (in mL or cm3 ) is multiplied by the density ( g/mL or g/cm3 ) the volume units will cancel out, leaving only the mass units. Keep in mind that the volume and density must use the same volume unit for the cancellation.
If a large marshmallow has a volume of 2.75 in3 and density of 0.242 g/cm3 , how much would it weigh in grams? 1 in3=16.39 cm3 .
Answers
The mass of the marshmallow is 10.9 g.
What is the relationship between mass, volume and density of a substance?The mass of a substance is the quantity of matter present in that substance.
The volume of a substance is the amount of space a given mass of that substance occupies.
The density of a substance is the ratio of the mass and a given volume of that substance.
Therefore, the relationship between mass, volume and density is given below as:
Density = mass/ volumeFrom the data provided:
volume of the marshmallow = 2.75 in³
1 inch³ = 16.39 cm³
volume in cm³ = 16.39 * 2.75
volume of the marshmallow = 45.07 cm³
density of the marshmallow = 0.242 g/cm³
Mass of the marshmallow = volume × density
Mass of the marshmallow = 0.242 g/cm³ * 45.07 cm³
Mass of the marshmallow = 10.9 g
Learn more about mass, volume and density at: https://brainly.com/question/952755
#SPJ1
A chemist decomposes samples of several compounds; the masses of their constituent elements are shown. Calculate the empirical formula for each compound. a. 1.651 g Ag, 0.1224 g O b. 0.672 g Co, 0.569 g As, 0.486 g O c. 1.443 g Se, 5.841 g Br
Answers
The empirical formula for each compound are as follow,
a)\(Ag_2 O\)
b)\(Co_3As_2O_8\)
c)\(SeBr_4\)
As per the data share in the above question are as follow,
The mass of their constituent elements are as follow,
a) Ag=1.651 g
O=0.1224 g
b)Co =0.672 g
As =0.569 g
O=0.486 g
c) Se =1.443 g
Br =5.841 g
We have to calculate the empirical formula for each compound.
a)Now Moles of (given mass upon Molar mass) \(Ag=\frac{1.651}{108} =0.015 \:moles\)
Moles of \(O=\frac{0.1224}{8\times 2} =0.0077 \:moles\)
Smallest mole value \(0.0077 \:moles\)
Divide all component by smallest mass.
Therefore \(Ag \Rightarrow\frac{0.015}{0.0077} \rightarrow2\\\\O \Rightarrow\frac{0.0077}{0.0077} \rightarrow1\)
Combine to get empirical formula,
\(Ag_2 O\)
b)Now Moles of (given mass upon Molar mass) \(Co=\frac{0672}{59} =0.011 \:moles\)
Moles of \(As=\frac{0.569}{75} =0.0076\: moles\)
Moles of \(O=\frac{0.486}{16} =0.0304 \:moles\)
Smallest mole value \(0.0076 \:moles\)
Divide all component by smallest mass.
Therefore
\(Co \Rightarrow\frac{0.011}{0.0076} \rightarrow1.5\rightarrow1.5 \times 2\rightarrow3\\\\As \Rightarrow\frac{0.0076}{0.0076} \rightarrow1\rightarrow1 \times 2\rightarrow2\\\\O \Rightarrow\frac{0.0304}{0.0076} \rightarrow4\rightarrow4 \times 2\rightarrow8\)
Combine to get empirical formula,
\(Co_3As_2O_8\)
c)Now Moles of (given mass upon Molar mass) \(Se=\frac{1.443}{79} =0.018 \:moles\)
Moles of \(Br=\frac{5.841}{80} =0.073 \:moles\)
Smallest mole value \(0.018 \:moles\)
Divide all component by smallest mass.
Therefore
\(Se \Rightarrow\frac{0.018}{0.018} \rightarrow1\\\\Br \Rightarrow\frac{0.073}{0.018} \rightarrow4\)
Combine to get empirical formula,
\(SeBr_4\)
For such more question on empirical formula.
https://brainly.com/question/1603500
#SPJ4
United Nations recommends a minimum of 50 litres of water per person per day.
Do all people get this much water? How can we find out that some places face shortage of water?
Answers
The World Health Organization (WHO) estimates that each individual needs between 50 and 100 litres of water per day to guarantee that their basic needs are met and that they have few health issues.
How much amount of water is recommended by the United Nations?For the majority of basic needs to be met and few health issues to materialise, the World Health Organization (WHO) states that between 50 and 100 litres of water per person per day are required.
The Assembly declared that every individual has the right to obtain enough water for personal and domestic needs, which equates to between 50 and 100 litres of water per person per day. The water must be suitable, acceptable, and reasonably priced.
A family is given three buckets of water each day. The 50 litres of water per person per day that the United Nations advises is. A family receiving three buckets of water per day does not indicate a water shortage.
To learn more about World Health Organization refer to:
https://brainly.com/question/20701235
#SPJ1
What is the “Island of Stability”?
Answers
Answer:
The island of stability is a term from nuclear physics that describes the possibility of elements with particularly stable "magic numbers" of protons and neutrons. This would allow certain isotopes of some transuranic elements to be far more stable than others, that is, decay much more slowly.
Explanation:
list five compound in chemistry and their formula
Answers
Answer:
Acetic acid CH3COOH
Hydrochloric acid HCl
Sulfuric acid H2SO4
Acetate CH3COO–
Ammonia
Explanation:
Hope i am right
A reaction occurs in a calorimeter, resulting in the starting temperature of 38.8 ℃ and final temperature 21.0 ℃. What can you say about the reaction and the enthalpy change (ΔH) during the reaction?
Answers
Answer:
hola comoe stas
Explanation:
gracias x los puntos
Discuss two pre-requisite skills needed for students to learn the process of writing balanced chemical and ionic equations
Answers
Answer:
Explanation:Two pre-requisite skills needed for students to learn the process of writing balanced chemical and ionic equations are:
1. Understanding of the periodic table and elements: Students must have a solid foundation in the periodic table, including recognizing elements by their symbols and understanding their properties, groups, and electron configurations.
2. Knowledge of chemical bonding and compound formation: Students should be familiar with the different types of chemical bonds (ionic, covalent, and metallic) and know how to construct chemical formulas for compounds based on their component elements and valence electrons.
Ben observes how quickly some dry wood is burning in a campfire. Which term best relates to Ben’s observation?
Answers
Answer:
Reaction rate
Explanation:
We are told that Ben observes how quickly some dry wood is burning in a campfire.
This is an example of reaction rate because it tells us the speed i.e. how fast the dry wood is reacting with the camp fire.
Assume that you measured exactly 2.0 mL of the sodium chloride solution into a small test tube. How many moles of
sodium chloride are in the tube?
Answers
Subscripts are the big numbers written to the left of a chemical formula in a chemical equation
True or false
Answers
Answer:
Most probably it's true.
what does celery, a wooden spoon, and oil/gasoline have in common?
Answers
Answer:
All of them are organic compounds which have carbon as their main atom in the structure.
Explanation:
Hello.
In this case, since organic chemistry is the study of all the compounds having the carbon atom as their main atom, all the vegetables, animals, an in general, living things are composed by lipids, proteins, and other organic substances with this feature. Moreover, wood-based materials are mainly composed by lignin which is an organic polymer also having carbon as the main atom. In addition, oil and gasoline are organic chemical compounds with a lot of applications in daily life which also contain carbon atoms in their structure.
In such a way, a celery, a wooden spoon, and oil/gasoline have the carbon atom in common as their main atom in their chemical structures.
Best regards.
A chemist uses a standard solution of 0.210 M lithium hydroxide (LiOH) to titrate 28.10 mL of sulfurous acid acid (H2SO3), she finds that it requires 22.14 mL of the base to reach the end-point of the titration. What is the molarity of the acid solution? What is the concentration of H2SO3?
Your answer must be rounded to the correct number of significant figures. Be sure to specify a unit.
Answers
Answer:
0.0827M of H₂SO₃
Explanation:
LiOH reacts with H₂SO₃ to produce water and Li₂SO₃, thus:
2LiOH + H₂SO₃ → 2H₂O + Li₂SO₃
Where 2 moles of lithium hydroxide react with 1 mole of sulfurous acid.
As the chemist requires 22.14mL = 0.02214L of a 0.210M solution to neutralize the acid, moles of LiOH are:
0.02214L × (0.210mol / L) =0.004649 moles of LiOH.
As 2 moles of LiOH react with 1 mole of H₂SO₃, moles of H₂SO₃ are:
0.004649 moles of LiOH ₓ (1 mole H₂SO₃ / 2 mol LiOH) =
0.002325 moles of H₂SO₃
These moles are present in 28.10mL = 0.02810L. Thus, molar concentration of the acid is:
0.002325 moles H₂SO₃ / 0.02810L = 0.0827M of H₂SO₃
Answer:
\(M_{acid}=0.0827M\)
Explanation:
Hello,
In this case, we should consider the acid-base reaction between sulfurous acid and lithium hydroxide:
\(2LiOH+H_2SO_3\rightarrow Li_2SO3+2H_2O\)
Thus, we notice a 2:1 molar ratio between lithium hydroxide and sulfurous acid, for that reason, at the equivalence point we have:
\(2*n_{acid}=n_{base}\)
That in terms of concentrations and volumes is:
\(2*M_{acid}V_{base}=M_{base}V_{base}\)
Thus, we solve for the molarity of the acid which is sulfurous acid:
\(M_{acid}=\frac{M_{base}V_{base}}{2*V_{base}} =\frac{0.210M*22.14mL}{2*28.10mL}\\\\M_{acid}=0.0827M\)
Best regards.
17. Consider the reaction shown and identify the statement that is not true.
825°C
CaCO3(s)
+ CaO(s) + CO2(g)
a.
This reaction is balanced as written.
b. The reactant must be heated for this reaction to occur.
c. The products are a solid and a gas.
d. Water must be present for this reaction to occur.
There are no solutions used in this reaction.
e.
Answers
Answer: Water must be present for this reaction to occur.
Explanation:
Decomposition reactions require breaking of bonds which require energy and thus all of the decomposition reactions are endothermic reactions.
The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) whereas liquids are represented by (l) and gases are represented by (g) after their chemical formulas.
The balanced chemical reaction of \(CaCO_3\) decomposition is:
\(CaCO_3(s)\rightarrow CaO(s)+CO_2(g)\)
The decomposition of \(CaCO_3\) requires heat and leads to formation of CaO as solid and \(CO_2\) as product.
Thus the the statement that is not true is Water must be present for this reaction to occur.
Which substance is completely consumed in a chemica reaction? limiting reactant reactant product
Answers
Answer:
Limiting reactant
Explanation:
The limiting reactant is completely is completely consumed in a reaction since it's not in excess and does not give a good yield of the product hence an excess reactant must have reacted with limiting reactant.
In an oxoacid such as H2SO4, ionizable hydrogen atoms are those bonded to oxygen
a. True
b. False
Answers
Answer:
true
Explanation:
Oxoacids are oxygen containing acids .The ionizable hydrogens in sulphuric acid are those bonded to the oxygen atoms. Hence, the statement is correct.
What are oxoacids?According to Bronsted-Lowry concept acids are substances which can furnish H+ ions when dissolved in water or on ionization. Oxoacids contains oxygen along with hydrogen atoms.
Some elements have a number of oxoacids mainly for group 15 and 16 elements. Phosphorus and sulphur have 4-5 oxoacids which differ in the number of oxygen and hydrogens.Oxoacids contains the hydroxyl group OH and the naming of oxoacids is based on the number of OH groups.
Sulphuric acid is an oxoacid of Sulphur containing 2 OH groups and two double bonded oxygens. Where the hydrogen atoms can be donated to a base to form its ionizable form .
To find more on oxoacids, refer here:
https://brainly.com/question/28306033
#SPJ5
Consider the reaction A + B ? products
From the following data obtained at a certain temperature, determine the rate law, the order of the reaction, and calculate the rate constant k.
Experiment 1: [A] = 1.50 M; [B] = 1.50 M; Initial Rate = 3.20 x 10-1 M/s
Experiment 2: [A] = 1.50 M; [B] = 2.50 M; Initial Rate = 3.20 x 10-1 M/s
Experiment 3: [A] = 3.00 M; [B] = 1.50 M; Initial Rate = 6.40 x 10-1 M/s
Please explain to me how you got the answer step by step >< Thank you!
A. Rate = k[A][B]
order of reaction = 2
k = 0.142 s-1
B. Rate = k[A]
order of reaction = 1
k = 0.213 s-1
C. Rate = k[A]2
order of reaction = 2
k = 0.142 s-1
D. Rate = k[B]
order of reaction = 1
k = 0.213 s-1
Answers
Answer:
B. Rate = k[A]
order of reaction = 1
k = 0.213 s-1
Explanation:
Hello,
In this case, you should consider the experiments and how the concentration of both A and B affect the rate, thus, we can make some conclusions:
- Between the experiment 1 and 2, we can notice that modifying the concentration of B does not affect the rate as it remains in 3.20 x 10-1 M/s, for that reason the reaction is zeroth-order respect to B.
- Between the experiment 2 and 3, we can notice that doubling the concentration of A from 1.50 M to 3.00 M results in a doubling of the rate from 3.20 x 10-1 M/s to 6.40 x 10-1 M/s, for that reason, we can infer that the reaction is first-order respect to A.
In such a way, we can infer that the rate law is:
\(Rate=k[A]^1[B]^0\\\\Rate= k[A]\)
Thus, the order of reaction is first-order and the rate constant turns out:
\(k=\frac{Rate}{[A]}=\frac{3.2x10^{-1}M/s}{1.50M}\\ \\k=0.213s^{-1}\)
Therefore, answer is:
B. Rate = k[A]
order of reaction = 1
k = 0.213 s-1
Regards.
help me i need it asap
Which example is a biotic factor of an aquarium ecosystem?
plastic plants placed in the gravel
algae growing on the glass
rock structure
gravel on the bottom of the aquarium
Which examples are an abiotic factor of an aquarium ecosystem? Select all that apply.
rock structure
temperature of the water
amount of algae on the glass
number of fish
Which example describes an abiotic factor interacting with a biotic factor?
The temperature of the air affecting the wind direction
Water eroding a rock
Cows eating nearby grass
The amount of sunlight affecting the growth of a plant.
Students in Ms. Brown's class are making observations in the garden outside of the school. They make a list of all the abiotic and biotic factors in their notebook. Part of the list is below:
Gravel
Grass
Butterfly
Sunflower
Bird feeder
Flowerpots
Which items on the list are a part of the garden ecosystem?
Grass, butterfly, sunflower
None of the listed items are a part of the ecosystem
All the listed items are part of the ecosystem
Gravel, bird feeder, flowerpots
Answers
The biotic factor of an aquarium ecosystem is algae growing on the glass.
The abiotic factors of an aquarium ecosystem are rock structure, temperature of the water, and amount of sunlight.
The example that describes an abiotic factor interacting with a biotic factor is the amount of sunlight affecting the growth of a plant.
All the listed items in Ms. Brown's class, including gravel, grass, butterfly, sunflower, bird feeder, and flowerpots, are a part of the garden ecosystem.
What is an ecosystem?An ecosystem is a community of living organisms (biotic factors) interacting with each other and with their physical environment (abiotic factors) in a specific area. It includes all the living and non-living components of the environment that interact with each other. Examples of ecosystems include a coral reef, a forest, a desert, and even an aquarium or a garden. Ecosystems can be large or small and can be found on land or in water. They are essential for the survival of living organisms as they provide food, shelter, and other resources necessary for life.
Read more on ecosystem here:https://brainly.com/question/842527
#SPJ1
NEVERMINDDDDDDDDDDDDDDD
Answers
Answer:
what is this free
branliest?
You have 0.672 L of 4.78 M aqueous AlCl3 solution in a glass. If you gently heat the solution until only 0.380 L is left, what is the new molarity of the AlCl3 solution?
Answers
Answer:
8.45 M
Explanation:
To solve this problem we need to keep in mind the definition of molarity:
Molarity = moles / volumeFirst we calculate the moles of AlCl₃ in 0.672 L of a 4.78 M solution:
Moles = Molarity * volumeMoles = 4.78 M * 0.672 L Moles = 3.212 molesThen we calculate the new molarity of the AlCl₃ solution using that number of moles, which remains the same throughout the evaporation process:
New Molarity = 3.212 moles / 0.380 LNew Molarity = 8.45 M1. What is the modern view of electrons in the quantum mechanical model?
Answers
Answer: An electron con only exist in a limited number of quantized energy levels.
Explanation:
Although chemical digestion occurs throughout the digestive system, it mostly occurs in the: Aesophagus and stomach B) liver and gall bladder liver and pancreas D stomach and intestines
Answers
Answer:
The digestive role of the liver is to produce bile and export it to the duodenum. The gallbladder primarily stores, concentrates, and releases bile. The pancreas produces pancreatic juice, which contains digestive enzymes and bicarbonate ions, and delivers it to the duodenum.
Explanation:
The chemical process of digestion begins during chewing as food mixes with ... The esophagus is a tubular organ that connects the mouth to the stomach. ... Acid reflux or “heartburn” occurs when the acidic digestive juices escape into the esophagus. ... The secretions of the liver, pancreas, and gallbladder are regulated by ...
-Both our right just choose one,
Consider the two molecules whose structures are shown below.
Which one would you expect to be more water soluble.
Explain your answer.
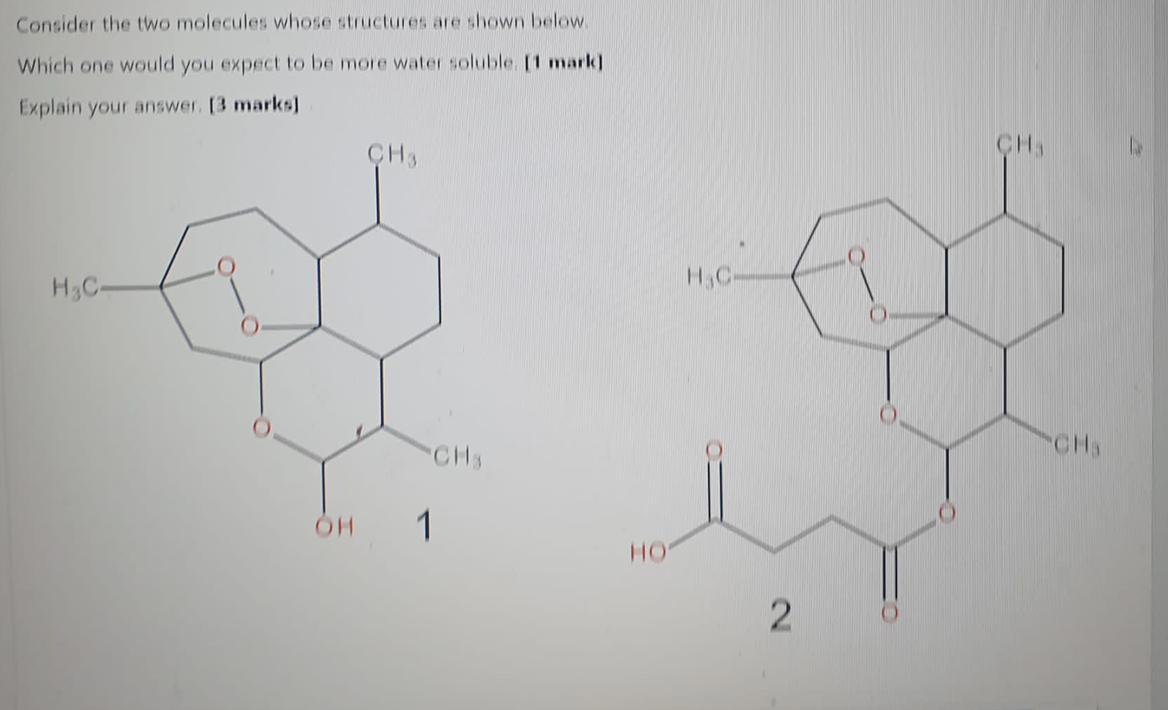
Answers
Molecule 1 will be more water soluble due to the presence of hydroxyl group.
Water solubilityMolecule 1 containing -OH (hydroxyl) will likely be more water-soluble compared to molecule 2 containing -COOH (carboxyl).
The hydroxyl group (-OH) forms hydrogen bonds with water molecules, promoting solubility. These hydrogen bonds allow for strong interactions between the polar hydroxyl group and the polar water molecules.
On the other hand, the carboxyl group (-COOH) is less soluble in water due to its additional carbonyl group (-C=O), which is less capable of forming hydrogen bonds.
Consequently, the presence of the carboxyl group reduces the overall solubility of the molecule compared to the hydroxyl group.
More on water solubility can be found here: https://brainly.com/question/29856613
#SPJ1
Question 10 of 10
Stacy set up three vials on a hot plate. He poured the same amount of liquid
into each of the vials, and then he turned on the hot plate. Which physical
property is he most likely testing?
OA. Hardness
OB. Surface tension
O C. Boiling point
OD. Melting point
Answers
Answer:C
Explanation:
C. Boiling point
Stacy is most likely testing the boiling point of the liquid in each vial. By heating the vials on the hot plate, he is increasing the temperature of the liquid and observing when it begins to boil. Boiling point is a physical property of a substance that is affected by factors such as pressure and temperature. It is the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by its surroundings. Therefore, by testing the boiling point of the liquid, Stacy can determine its identity or purity.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
11) Quique mixed 100. milliliters of 1.0 M BaCl₂(aq) and 200. milliliters of 0.5 M Na2SO4(aq) in a coffee cup calorimeter at 45.0°C. The solutions reactec
BaCl₂(aq) + Na₂SO4(aq)
BaSO4(s) + 2 NaCl(aq)
During the reaction, the temperature of the water in Quique's calorimeter dropped to 15.8°C. What is the heat of this endothermic reaction?
-
A)-366 kJ/mol BaCl₂
B)-198 kl/mol BaCl₂
C) 198 kl/mol BaCl₂
D) 366 kl/mol BaCl₂
Answers
The heat of reaction per mole of BaCl₂ is 366 kJ/mol BaCl₂.
What is reaction?Reaction is a response, typically sudden, to a particular event, situation, or stimulus. It is a type of behavior that occurs as a result of an external force, such as an event, person, or object. Reactions can be physical, emotional, mental, or a combination of all three. Examples of physical reactions include facial expressions, muscle tension, and changes in heart rate and breathing.
The heat of this endothermic reaction can be calculated using the equation q=mc∆T,
where q is the heat,
m is the total mass of the solution,
c is the specific heat capacity of water, and
∆T is the change in temperature.
In this case, the total mass of the solution is 300 mL and the change in temperature is (45.0°C - 15.8°C) = 29.2°C.
The specific heat capacity of water is 4.18 J/g°C.
Therefore, the heat of the reaction is q = (300 mL)(4.18 J/g°C)(29.2°C) = 366 kJ.
Since the reaction is 1 mole of BaCl₂,
The heat of reaction per mole of BaCl₂ is 366 kJ/mol BaCl₂.
To learn more about reaction
https://brainly.com/question/16416932
#SPJ1
Why does it mean by methane molecule is symmetrical?
Answers
A methane molecule (CH4) is considered symmetrical because it possesses a symmetric structure and exhibits symmetry operations.
Symmetry refers to a balanced arrangement of elements that can be divided into equal parts by a plane, axis, or center. In the case of methane, it exhibits several symmetrical characteristics.
Firstly, methane has a tetrahedral molecular geometry, with the carbon atom at the center and four hydrogen atoms positioned around it. This geometry ensures that the molecule is symmetrical in terms of its spatial arrangement.
Each hydrogen atom is located at one of the vertices of the tetrahedron, forming equal angles and distances with respect to the central carbon atom. This symmetry is maintained regardless of the orientation of the molecule.
Additionally, methane possesses rotational symmetry. It can be rotated around any of the carbon-hydrogen bonds, and the molecule will retain its overall appearance.
The symmetry of methane arises from its molecular structure and the equal distribution of electron density around the central carbon atom. The four hydrogen atoms are bonded to the carbon through sigma bonds, which have a cylindrical symmetry. This balanced arrangement of the atoms contributes to the overall symmetry of the molecule.
For more such questions on methane visit:
https://brainly.com/question/25207057
#SPJ8