Answers
Answer:
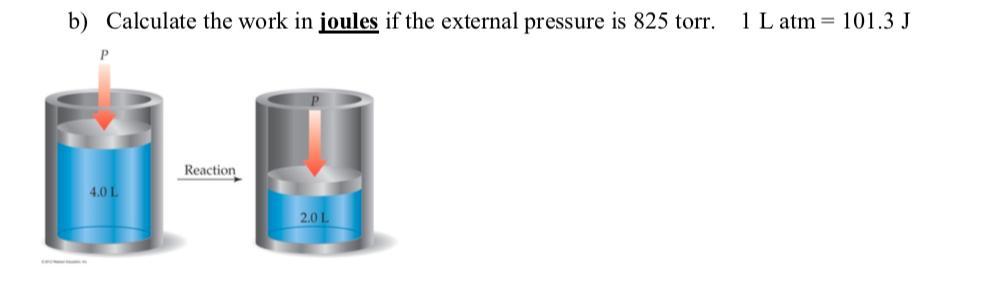
\(\large \boxed{\text{220 J}}\)
Explanation:
1. Convert the pressure to atmospheres
\(p = \text{825 Torr} \times \dfrac{\text{1 atm}}{\text{760 Torr}} = \text{1.086 atm}\)
2. Calculate the work done
\(w = -p\Delta V = -p(V_{f} - V_{i}) = -\text{(1.086 atm)}(\text{2.0 L - 4.0 L})\\\\ = -\text{1.086 atm} \times \text{(-2.0 L)} = \text{2.17 L$\cdot$atm}\)
3. Convert litre atmospheres to joules
\(w = \text{2.17 L$\cdot$atm} \times \dfrac{\text{101.3 J}}{\text{1 L$\cdot$atm}} = \textbf{220 J}\\\\\text{The work done is $\large \boxed{\textbf{220 J}}$}\)
Note that the volume is decreasing, so work is being done on the system.
Related Questions
( Endocytosis / Exocytosis ) is the movement of substances out of a cell by vesicular transport.
Answers
Answer:
Exocytosis
Explanation:
Some molecules are simply too big to move via a transport protein or the plasma membrane. To carry these macromolecules in or out of the cell, cells employ two more active transport pathways. Macromolecules or big particles are transported across the plasma membrane via Vesicles transport or other cytoplasmic structures. They are of two types, Endocytosis and Exocytosis
From the given information, Exocytosis is the right answer.
It is the process of vesicles combining with the plasma membrane thereby releasing their contents to the exterior of the cell. When a cell creates components for export, such as proteins, or when it gets rid of a waste product or a toxin, exocytosis occurs. Exocytosis is the process by which newly generated membrane proteins and membrane lipids are transported on top of the plasma membrane.
. Critique Reasoning Maddy wants to know how
many centigrams are in 0.75 gram. She converted
0.75 gram to its equivalent in centigrams as
shown. Is her work correct? Explain.
10 cg x 0.75
1gx 0.75
=
7.5 cg
0.75 g
Answers
Maddy made the error of multiplying by 10 rather than 100, which produced an answer that was 10 times off in the conversion of units.
The work of Maddy is flawed. We must multiply the value in grammes by 100 to convert it to centigrammes. Maddy gave the wrong answer of 7.5 cg by multiplying the weight in grammes by 10 rather than 100.
In order to convert 0.75 grammes to centigrammes, use the following formula:
100 cg/g x 0.75 g equals 75 cg
Consequently, 0.75 grammes is 75 centigrammes. Maddy made the error of multiplying by 10 rather than 100, which produced an answer that was 10 times off.
Learn more about conversion of units:
https://brainly.com/question/19420601
#SPJ1
4. A balloon filled with 2 L of air is at 313 K. Suppose the temperature increases to 600 K. Calculatewhat happens to the volume of the balloon.
Answers
According to Charle's law, a balloon at 600 K has a volume of 3.83 L.
Describe Charles's law.Charles' law states that, under constant pressure, the volume a given amount of gas fills is directly proportional to its absolute temperature. For instance, when the temperature drops during the winter, a basketball placed outside in the ground would contract.
Mathematically, Charles' law can be written as V1/T1 = V2/T2, where V1 denotes the volume at the beginning, T1 the temperature at that point, and V2 the volume at the end with T2 the temperature at that point.
The following examples of how Charles' law is applied in real life:
Helium balloons shrink when it's cold outside.
From Charles' law, V/T = constant
V₁/T₁ = V₂/T₂
or, 2/313 = V₂/600
or, V₂ = (2x600)/313
= 3.83
Hence, form Charles' law we conclude the volume of balloon at 600 K is 3.83 L.
To know more about Charles' law, click on the link
https://brainly.com/question/16927784
#SPJ10
Which statement accurately describes the atoms of a specific element? *
An indium, In, atom contains 115 protons inside the nucleus and 49 neutrons outside the nucleus.
A scandium, Sc, atom contains 45 electrons outside the nucleus and 21 neutrons inside the nucleus.
An aluminum, Al, contains 27 electrons and 27 protons inside the nucleus.
A zinc, Zn, atom contains 30 protons inside the nucleus and 30 electrons outside the nucleus
Answers
Answer:
the last one, (A zinc, Zn, atom contains 30 protons inside the nucleus and 30 electrons outside the nucleus)
A zinc, Zn, atom contains 30 protons inside the nucleus and 30 electrons outside the nucleus accurately describes the atoms of a specific element ,therefore option (d) is correct.
What are the characteristics of zinc element ?Zinc is placed in group 12 of the periodic table. It is in the first period of transition or d block elements, and fourth period of the periodic table.
Zinc is a metal. It has two valence electrons that are lost easily. Zinc forms positive ions with 2+ charges, like the metals of the alkaline earth family.
Uses of zinc element -:
Zinc is an important trace element for human beings. Zinc is used for making brass, an alloy of copper and zinc.Zinc is used as the anode in dry cells.A zinc, Zn, atom contains 30 protons inside the nucleus and 30 electrons outside the nucleus accurately describes the atoms of a specific element ,hence option (d) is correct.
Learn more about zinc element ,here:
https://brainly.com/question/7242563
#SPJ6
What is the value of Ksp for Ag2SO4 if 5.40g is soluble in 1.00 L of water?
Answers
The expression for the equilibrium constant is Ksp (= [Ag+][I-] = (x)(x) = x2. Step 3: Use the solubility of AgI to calculate the molar solubility. At 100oC, silver sulphate dissolves in water at a rate of around 1. 4 grams per mL.
How should a Ksp equation be written?The equilibrium formula, Ksp, can be expressed as Ksp (= [products]/[reactants] and represents a ratio of products to reactants. Equilibrium balance between a cationic solid as well as its solution's ions is shown by this statement.
What is the Ksp equivalent of solubility?The greater a substance's K s p chemical value, the more soluble it is. What exactly are K sandeep units. In actuality, it lacks a unit! Since the molar quantities of the products and reactants vary for each equation, the K s p value lacks units.
To know more about molar visit:
https://brainly.com/question/8732513
#SPJ1
What is the density of a sample if its mass is 44.3g and its volume is 22.1cm?
Answers
Answer:
about 2 g/cm
Explanation:
D = M/V
D = 44.3/22.1
D ≈ 2
Pls mark Brainliest with the crown
the role of haemoglobin in the transport of oxygen and carbon dioxide
Answers
Hemoglobin with bound carbon dioxide and hydrogen ions is carried in the blood back to the lungs, where it releases the hydrogen ions and carbon dioxide and rebinds oxygen. Thus, hemoglobin helps to transport hydrogen ions and carbon dioxide in addition to transporting oxygen.
If you have 54.63g of TiCl4, determine the theoretical yield of TiO2.
480.90g TiO2
480.90g TiO, 2
23.00 g TiO2
23.00 g TiO, 2
30.06g TiO2
30.06g TiO, 2
1.11g TiO2
1.11g TiO, 2
Answers
Answer:
its D:)
Explanation:
What is this element help asap

Answers
Explanation:
In this element, there are:
11 protons (in blue)12 neutrons (in red), and11 electrons (in green)We find the element with atomic number 11, which is Sodium. (Na)
Answer: sodium
Explanation:
Any help?
The Kb for hydroxylamine, HONH2, is 1.1 x 10 -8
. What would be the pH of a solution
prepared by placing 1.34 g of HONH2 in 0.500 L of water?
Answers
Answer:
pH = 9.475
Explanation:
Hello there!
In this case, according to the basic ionization of the hydroxylamine:
\(HONH_2+H_2O\rightarrow HONH_3^++OH^-\)
The resulting equilibrium expression would be:
\(Kb=\frac{[HONH_3^+][OH^-]}{[HONH_2]} =1.1x10^{-8}\)
Thus, we first need to compute the initial concentration of this base by considering its molar mass (33.03 g/mol):
\([HONH_2]_0=\frac{1.34g/(33.03g/mol)}{0.500L} =0.0811M\)
Now, we introduce \(x\) as the reaction extent which provides the concentration of the hydroxyl ions to subsequently compute the pOH:
\(1.1x10^{-8}=\frac{x^2}{0.0811-x}\)
However, since Kb<<<<1, it is possible to solve for \(x\) by easily neglecting it on the bottom to obtain:
\(x=[OH^-]=\sqrt{1.1x10^{-8}*0.0811}= 2.99x10^{-5}\)
Thus, the pOH is:
\(pOH=-log(2.99x10^{-5})=4.525\)
And the pH:
\(pH=14-4.525\\\\pH=9.475\)
Regards!
When mining occurs what is a significant direct impact on the environment?
More construction can happen with the metals that are mined, which can disrupt new ecosystems
Fossil fuels burned during mining release carbon dioxide, leading to ocean acidification
There are more resources used to recycle the metals that are mined.
Habitat is removed for wildlife that used the hills and mountains where mines are built.
Answers
Answer:
More construction can happen with the metals that are mined, which can disrupt new ecosystems
Fossil fuels burned during mining release carbon dioxide, leading to ocean acidification
Explanation:
Good Luck!
answer: the answer is More construction can happen with the metals that are mined, which can disrupt new ecosystems.
Explanation:
since he wana give 2 dang on answers to a 1 answer question like dang!!
How many moles of sodium hydrogen carbonate are present in 2.61 grams of this compound?
Answers
Molar mass of NaHCO_3
23+1+12+3(16)36+4884g/molNow
\(\\ \sf\longrightarrow No\:of\:moles=\dfrac{Given\:mass}{Molar\:mass}\)
\(\\ \sf\longrightarrow No\:of\; moles=\dfrac{2.61}{84}\)
\(\\ \sf\longrightarrow No\:of\:moles=0.0312mol\)
why does the ratio of chloride ions to calcium ions is 2:1 when calcium chloride forms
Answers
how many formula units are in 62.2 g of aluminum sulfide
Answers
2.49 * 10^23 formula units make up a sample of aluminum sulfide that weighs 62.2 g.
Describe a mole.
A mole is 6.022 * 10^23 atoms, molecules, ions, or other particles.
The number of formula units in the 62.2 g sample of aluminum sulfide is specified in the question.
Calculating the number of moles is necessary to get the number of formula units.
Aluminum Sulfide has a molecular weight of 150.158 gm.
1 mole is made up of 150.158 g of aluminum sulfide.
62.2 g will yield 0.414 moles when multiplied by 1 / 150.158.
Formula units: 6.022 * 10^23 equals one mole.
0.414 moles = 6.022 * 10²³ *0.414
= 2.49 x 10^23 mathematical units.
A 62.2 g sample of aluminum sulfide therefore has 2.49 * 10^23 formula units.
To know more about aluminum sulfide, click on the link below:
https://brainly.com/question/27839950
#SPJ9
62.2 percent Hf by mass and 37.4 percent Cl by mass. What is the empirical formula for this compound?
Answers
Answer:
HfCl3
Explanation:
Step 1: Find the compounds relative atomic mass
Step 2: Divide the percentage given by the relative atomic mass
Hf = 62.2/178.5 CI = 37.4/35.5
Hf = .348 CI = 1.054
Step 3: Divide every given number by its lowest ratio. (0.348)
Hf - .348/.348 CI - 1.054/.348
Hf= 1 CI = 3
This means, the empirical formula for this compound would end up being
HfCI3.
Hope this helps!
In this lab you will need to prepare solutions using dilutions. Starting with the stock 0.300 M NaOH solution, how would you prepare a 0.100 M NaOH solution (using 0.300 M NaCl as the diluent)
Answers
Answer:
1/3 dilution : 1 volume of stock solution + 2 volumes of diluent
Explanation:
Concentrated solution = 0.300 NaOH
Diluted solution = 0.100 NaOH
The dilution factor is : 0.100/0.300 = 1/3
Thus, we have to dilute three times the stock solution. For this, we take 1 volume of stock solution and then we add 2 volumes of diluent. As result, we will have 1 volume of stock solution in a total of 3 volumes. For example, we take 1 mL of 0.300 NaOH and then we add 2 mL of diluent (0.300 NaCl). The final concentration after dilution will be:
Cd = 0.300 M x 1 mL/3 mL = 0.100 M
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
why blood is separated into different parts
Answers
Answer:
Blood fractionation is the process of fractionating whole blood, or separating it into its component parts. This is typically done by centrifuging the blood. The resulting components are: a clear solution of blood plasma in the upper phase (which can be separated into its own fractions, see Blood plasma fractionation),
Answer: Centrifugal force is used to separate the components of blood – red blood cells, platelets and plasma – from each other. ... The red blood cells precipitate to the bottom of the bag, with the platelets above them, then the white blood cells and the plasma at the very top. Also because Each part of the blood has a different function. Separating the blood into parts lets patients get only the specific part or parts of the blood that they need. So a whole blood donation can be used for several patients.
Hope this helps have a awesome day/nigh❤️✨t
Explanation:

list several characteristics of ligroin
Answers
- Petroleum fraction consisting mostly of \(C_{7}\) and \(C_{8}\) hydrocarbons
- The fraction is also called heavy naphtha
- Boiling in the range 90‒140 °C
- Ligroin is used as a laboratory solvent
- Ligroin it also esed as a motor fuel or as a solvent for fats and oils in dry cleaning
[] All of these are different characteristics of ligroin
[] If you need more let me know
Have a nice day!
I hope this is what you are looking for, but if not - comment! I will edit and update my answer accordingly. (ノ^∇^)
- Heather
Question 6 (1 point)
"When two or more objects collide, there will be the same amount of momentum before the collision as after" is
a) Momentum Rule
b) Law of Collisions
c) Law of Conservation of Momentum
d) The Law of Before and After
Answers
Give the name of the ion with 13 protons and 10 electrons
Answers
Answer:
Explanation:
aluminum
Answer: The aluminum ion
Explanation:
write electron dot structures for the atoms and ions of each of the following elements. 1. ca 2. br 3. al
Answers
With a 20 atomic number and 20 electrons, calcium is an atom. The atom of bromine has an atomic number of 35 and 35 electrons. 13 electrons make up the atom of aluminium, which has an atomic number of 13.
Write electron dot structures for ca. br. al. ?Calcium: The atom of calcium contains 20 electrons and an atomic number of 20. Calcium has an electron dot structure of 2,8,8,2. Accordingly, the first shell contains two electrons, the second shell eight, the third shell eight, and the fourth shell two electrons.Bromine: The atom of bromine contains 35 electrons and an atomic number of 35. Bromine has an electron dot structure of 2,8,18,7. Accordingly, the first shell contains two electrons, the second shell eight, the third shell eighteen, and the fourth shell seven.Aluminum: The atom of aluminium contains 13 electrons and an atomic number of 13. Aluminum has an electron dot structure of 2,8,3. Accordingly, the first shell contains two electrons, the second shell eight, and the third shell three.Ca: [Ar] 4s2.Br: [Ar] 3d10 4s2 4p5.Al: [Ne] 3s2 3p1.To learn more about electron dot structures for the atoms and ions refer to:
https://brainly.com/question/24774222
#SPJ4
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
A fuel tank holds 22.3 gallons of gasoline. If the density of gasoline is 0.8206 g/mL what is the mass in Kg of gasoline in a full tank?
Answers
The mass of gasoline in the full tank is 68.99 kilograms. Gasoline is a highly flammable and volatile liquid that can easily ignite if exposed to heat or a spark.
What is Gasoline?
Gasoline, also known as petrol, is a transparent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It is a mixture of hydrocarbons, typically containing 5 to 12 carbon atoms per molecule. Gasoline is refined from crude oil through a process of distillation, whereby the crude oil is heated and separated into its different components based on their boiling points.
First, we need to convert the volume of gasoline from gallons to milliliters:
1 gallon = 3.78541 liters
1 liter = 1000 milliliters
Therefore:
22.3 gallons x 3.78541 liters/gallon x 1000 milliliters/liter = 84,161.83 milliliters
Next, we can use the density of gasoline to find the mass of the gasoline:
0.8206 g/mL x 84,161.83 mL = 68,986.87 grams
68,986.87 grams / 1000 grams per kilogram = 68.99 kilograms (rounded to two decimal places)
Therefore, the mass of gasoline in the full tank is 68.99 kilograms.
Learn more about Gasoline from given link
https://brainly.com/question/25736513
#SPJ1
Answer : 69.3 kg
Explanation:
If AB = 5 inches and AD = 8, find BD. Round to the nearest tenth if necessary.
WILL GIVE BRAINLIEST
Answers
Answer:
6.5
Explanation:
half of 5 is 2.5, half of 8 is 4. 2.5+4=6.5
:)
The valves in the heart open and close to move blood in between heart chambers. O a) three directions O b) two directions O c) four directions d) one direction
Answers
Answer:
im not sure but i think its four directions
Hi,
The valves in the heart open and close to move blood in between heart chambers.
Answer:
d) one direction
Objects that have a higher density than water can sometimes be observed floating on water. which property of water explains this phenomenon?
Answers
Answer: Under what condition an object having density greater than water will float on water?
If an object is more dense than water it will sink when placed in water, and if it is less dense than water it will float. Density is a characteristic property of a substance and doesn't depend on the amount of substance.
if you don't stan Tomo Yamanobe, you aren't living :D

Answers
Answer: nice, is this a quistion? xd
Explanation:
Answer:
O-O
Explanation:
mmmmmmmmmmmmmmmmmmmmmmmmmmm
Explain how temperature and air pressure play a role in creating wind.
Answers
How temperature and air pressure play a role in creating wind.
Gases move from high-pressure areas to low-pressure areas. And the bigger the difference between the pressures, the faster the air will move from the high to the low pressure. That rush of air is the wind we experience.
hope it helps.
Aliyyah.
Winds are created by the change in temperature gradient which results in the difference in pressure causes the circulation of air from high pressure area to low pressure area that is called winds.
What is winds?Wind is the circulation or passage of air from one region to the other over a pressure gradient. The pressure gradient is resulting from the uneven heating of the atmosphere.
According to Gay- Lussac's law, the pressure of a gas is directly proportional to the temperature of the region. Thus as the temperature over an area increases pressure also increases.
The heat radiated from the sun is unevenly distributed in the earth atmosphere results in difference in pressure also. To balance this pressure difference, the air from high pressure region passes to low pressure region creating the winds.
To find more on winds, refer here:
https://brainly.com/question/12005342
#SPJ2
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100