Which statements below are true?Check all that apply.A. Distance measurements must include magnitude, unit, anddirection.B. Displacement can be fully described with a magnitude and a unit.C. If motion starts and stops at the same location, then thedisplacement is zero.D. Distance is always greater than or equal to the magnitude of medisplacement.
Answers
INFORMATION:
We have the next statements:
A. Distance measurements must include magnitude, unit, and direction.
B. Displacement can be fully described with a magnitude and a unit.
C. If motion starts and stops at the same location, then the displacement is zero.
D. Distance is always greater than or equal to the magnitude of me displacement.
And we must determine which are true
STEP BY STEP EXPLANATION:
To determine which statements are true, we need to analyze each one:
A. Distance measurements must include magnitude, unit, and direction.
The distance between two points in three-dimensional space is a scalar. So, the distance is represented only by
Related Questions
Based on the evidence collected in the activity where a corn chip was burned, what claim can you make about the relationship between burning food outside the body and food reacting with oxygen inside the body?
Answers
The evidence collected in the activity suggests that burning food outside the body is a form of oxidation, which is a reaction between food and oxygen inside the body. This reaction is responsible for the production of energy and the release of waste products.
What is a polar molecule?

Answers
Answer:
The answer is D
Explanation:
A polar molecule is when the electrons are not shared evenly, developing oppositely charged dipoles.
PLEASE HELP!!!!!!!!!!!
Molar mass is the molecular (formula) mass of any substance expressed in which unit?
kilograms
liters
moles
grams
Answers
Answer:
I believe your answer would be grams.
3. If an element has 47 protons and 54 neutrons what is the element and what is its atomic
mass?
4. If one atom has 47 neutrons and a mass of 87 and another one has 41 protons and a
mass of 87, are they isotopes of each other?
5. Draw the electron dot diagram for the element Phosphorous.
Answers
4.Nb(Niobium) the atomic mass is 92.906
5.

If you have 3.75 x 10^15 atoms of gold, how much gold do you have in grams? (gold = Au)
Answers
Answer:
1.23*10^-6 g Au
Explanation:
take your atoms and divide by Avogadro's number to get number of moles
3.75 x 10^15 / 6.022 x 10^23 = 6.227 x 10^-9 moles
multiple the moles by molar mass of Au to get grams Au
6.227 x 10^-9 moles * 196.966 = 1.226*10^-6
round to 3 sig fig
Please help me with this thank you I’ll give extra credit!

Answers
I have no answer for you because we don't have the text either
where do you need to orient your eye level to make accurate reading of the meniscus
Answers
One of the commercial uses of sulfuric acid is in the production of calcium sulfate and phosphoric acid. If 23.7 g of Ca₃(PO₄)₂ reacts with an excess of H₂SO₄, what is the percent yield if 13.4 g of H₃PO₄ are formed in the following UNBALANCED chemical equation
Answers
The percentage yield would be 90.42%
First, the balanced equation of the reaction is as follows:
Ca₃(PO₄)₂ (s) + 3H₂SO₄ (aq) → 2H₃PO₄ (aq) + 3CaSO₄ (aq)
From the equation, the mole ratio of Ca₃(PO₄)₂ and H₃PO₄ is 1:2
Recall that: mole= mass/molar mass
Mole of Ca₃(PO₄)₂ = 23.7/310.174
= 0.076 mole
Mole ratio of Ca₃(PO₄)₂ and H₃PO₄ = 1:2
Mole of H₃PO₄ = 0.076 x 2
= 0.153 mole
Theoretical yield (in g) of H₃PO₄ = mole x molar mass
= 0.153 x 97.994
= 14.82 g
Percentage yield of H₃PO₄ = actual yield/theoretical yield x 100
= 13.4/14.82 x 100
= 90.42%
More on percentage yield of reactions can be found here: brainly.com/question/20758645
If the pH of an acid rain storm is approximately 3.0, how many times greater is the [H ] in the rain than in a cup of coffee having a pH of 5.0
Answers
Taking into account the definition of pH, the [H⁺] in the rain is 100 times greater than in a cup of coffee.
Definition of pHpH is the Hydrogen Potential. It is a measure of acidity or alkalinity that indicates the amount of hydrogen ions present in a solution or substance.
Mathematically, pH is calculated as the negative base 10 logarithm of the activity of hydrogen ions:
pH= - log [H⁺]
The numerical scale that measures the pH of substances includes the numbers from 0 to 14. The pH value 7 corresponds to neutral substances. Acidic substances are those with a pH lower than 7, while basic substances have a pH higher than 7.
Comparison of [H⁺]In this case, you know:
pH= 3pH= 5You can replace this values in the definition of pH:
3= -log [H⁺] → [H⁺]=1×10⁻³ M5= -log [H⁺] → [H⁺]=1×10⁻⁵ MSolving:
[H⁺]=1×10⁻³ M[H⁺]=1×10⁻⁵ MComparing [H⁺], you get:
\(\frac{1x10^{-3} }{1x10^{-5} } =100\)
Finally, the [H⁺] in the rain is 100 times greater than in a cup of coffee.
Learn more about pH:
https://brainly.com/question/26424076
https://brainly.com/question/26524338
https://brainly.com/question/13557815
https://brainly.com/question/24595796
https://brainly.com/question/24753206
What is the molarity of HCl if 36.99 mL of a solution of HCl contain 0.3219 g of HCl?
Answers
what would the pressure be at 25.0g of chlorine gas at "-10.0celsius" in a 4.50 L
Answers
The pressure of the chlorine gas at the given condition is 1.7 atm.
What is the pressure of the chlorine gas?The pressure of the chlorine gas at the given condition is calculated by applying ideal gas law.
PV = nRT
where;
n is the number of molesR is the ideal gas constantT is the temperatureThe number of moles of 25 g of chlorine is calculated as follows;
n = m/M
n = 25/71
n = 0.352
The pressure of the chlorine gas at the given condition is calculated as;
P = nRT/V
P = (0.352 x 0.0821 x 263) / (4.5)
P = 1.7 atm
Learn more about pressure of gas here: https://brainly.com/question/25736513
#SPJ1
8. Explain how Elianto oil is obtained from maize seeds.
Answers
Answer:
Almost all corn oil is expeller-pressed
Explanation:
then solvent-extracted using hexane or 2-methylpentane (isohexane). The solvent is evaporated from the corn oil, recovered, and re-used. After extraction, the corn oil is then refined by degumming and/or alkali treatment, both of which remove phosphatides.Oct 16, 2020
The ratio of the mass of O
to the mass of N
in N2O3
is 12:7. Another binary compound of nitrogen has a ratio of O
to N
of 16:7.
What is the ratio of O
to N
in the next member of this series of compounds?
Answers
much for the invite too but i just got home from work and i woke up N:14
what is a benefit of the artificial selection for the environment and explain it. please help!!
Answers
1x+cb=23
thios is right because math
A civil engineer designs mostly:
A. building structures.
B. computer parts.
C. new foods.
D. technology that flies.
Answers
DNA is said to be the organism's genetic fingerprint. What does it mean? Give one application of this concept
Answers
Answer:
DNA is an organism's genetic fingerprint because it contains unique genetic information that determines its development, function, and reproduction. This unique DNA sequence can be used for identification purposes, such as establishing paternity or maternity, identifying suspects in criminal investigations, and identifying remains in forensic investigations. The field of forensic science uses DNA analysis to compare DNA profiles from crime scenes to those of potential suspects, resulting in the conviction of many criminals who would have otherwise gone unpunished.
A sample of ammonia, NH3, has a mass of 78.25 g. Calculate the number of ammonia molecules in the sample.
number of molecules:
Answers
There are approximately \(2.76 * 10^{24\) ammonia molecules in the given sample.
To calculate the number of ammonia molecules in the sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia \((NH_3)\) can be calculated by adding up the atomic masses of nitrogen (N) and hydrogen (H):
Molar mass of \(NH_3\) = (1 x atomic mass of N) + (3 x atomic mass of H)
= (1 x 14.01 g/mol) + (3 x 1.01 g/mol)
= 14.01 g/mol + 3.03 g/mol
= 17.04 g/mol
Now, we can calculate the number of moles of ammonia in the sample using the formula:
Number of moles = Mass of the sample / Molar mass
Number of moles = 78.25 g / 17.04 g/mol
≈ 4.5865 mol (rounded to four decimal places)
Finally, we can use Avogadro's number, which represents the number of particles (atoms, molecules, etc.) in one mole of a substance. Avogadro's number is approximately \(6.022 * 10^{23\) particles/mol.
Number of ammonia molecules = Number of moles x Avogadro's number
Number of ammonia molecules ≈ 4.5865 mol x (\(6.022 * 10^{23\) molecules/mol)
≈ \(2.76 * 10^{24\) molecules (rounded to two significant figures)
Therefore, the provided sample contains roughly \(2.76 * 10^{24\) ammonia molecules.
Learn more about moles on:
https://brainly.com/question/24748125
The number of ammonia molecules in the sample is approximately 2.764 x \(10^{24}\) molecules.
To calculate the number of ammonia molecules in a given sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia (NH3) is calculated as follows:
Molar mass of N = 14.01 g/mol
Molar mass of H = 1.01 g/mol
Total molar mass of NH3 = 14.01 g/mol + (3 * 1.01 g/mol) = 17.03 g/mol
Now, we can calculate the number of moles of ammonia in the sample:
Number of moles = Mass of sample / Molar mass of NH3
Number of moles = 78.25 g / 17.03 g/mol = 4.594 moles
Next, we use Avogadro's number, which states that there are 6.022 x \(10^{23}\) molecules in one mole of a substance.
Number of molecules = Number of moles * Avogadro's number
Number of molecules = 4.594 moles * 6.022 x \(10^{23}\) molecules/mol = 2.764 x \(10^{24}\) molecules
Therefore, there are approximately 2.764 x \(10^{24}\) ammonia molecules in the given sample of 78.25 g.
Know more about Avogadro's number here:
https://brainly.com/question/1513182
#SPJ8
Which of the following are units of pressure?

Answers
Answer:
atm
kPa
torr
mmHg
HELP ME OUT PLS!!!!!
A swimmer makes a turn at a pool wall.
Which answer choice best describes the direction the swimmer accelerates when she pushes against the wall?
A) Away from her push because the wall exerts an equal and opposite force
B) Away from her push because the wall exerts a stronger force in the same direction
C) Same direction as her push because the wall exerts an equal force in the same direction
D) Same direction as her push because the wall exerts a stronger and opposite force

Answers
Answer:
C
Explanation:
Well Its called reaction force, so that helps you figure out what direction she would go towards. When you use anything that has reaction force what way are they normally going? So thats why she stayed still.
Solve please
………………………………………
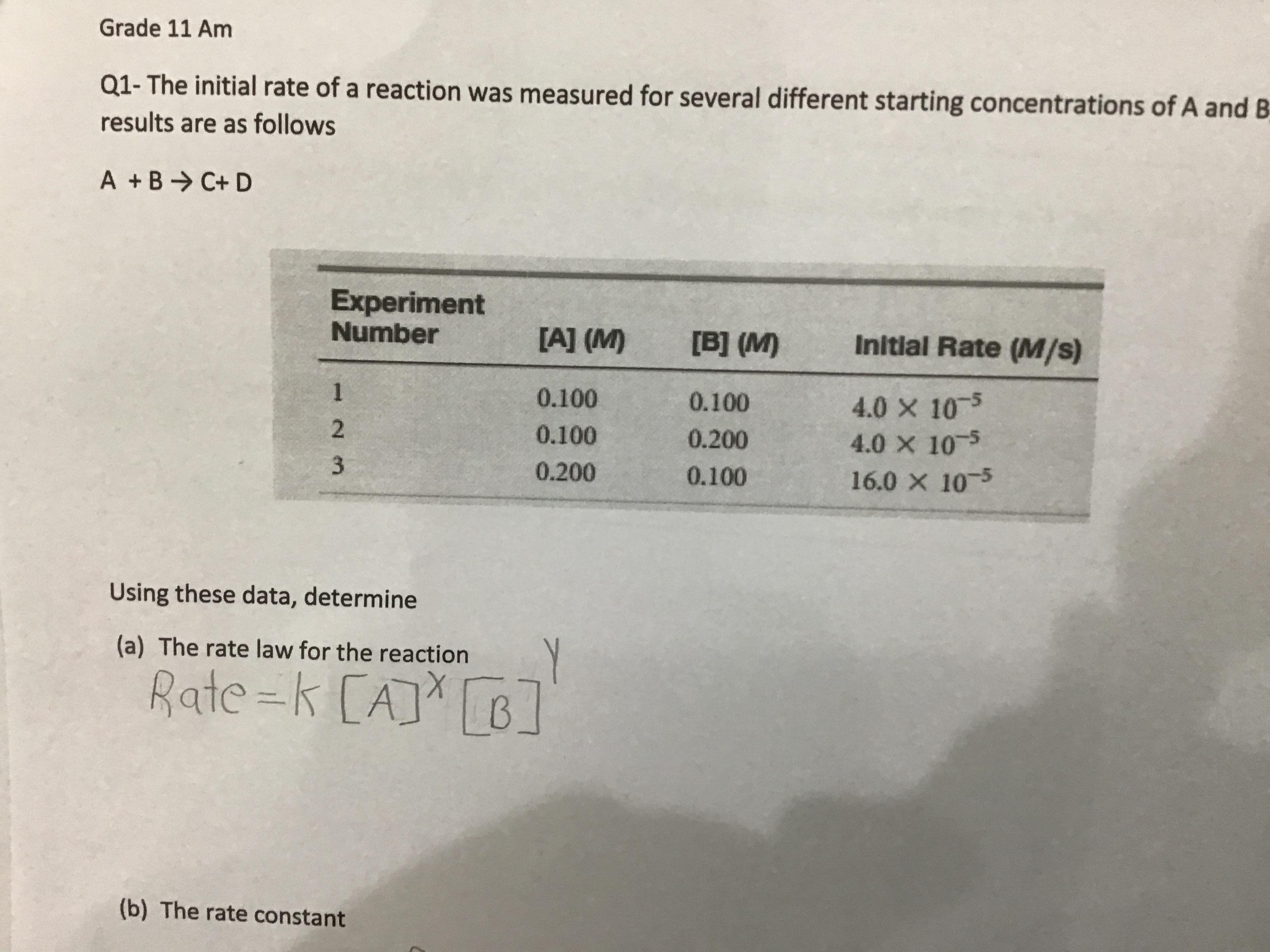
Answers
a) The rate law for reaction is Rate = k[A]² [B]⁰
b) the rate constant is k = 4 × 10⁻³
a) For the Rate law for reaction
the equation is given as:
A + B -----> C + D
Rate = k[A]^x[B]^y
in equation (1) and (2)
4.0 × 10⁻⁵ = k[0.1]^x[0.1]^y equation (1)
4.0 × 10⁻⁵ = k[0.1]^x[0.2]^y equation (2)
equation (2) / (1)
(2)⁰ = (2)^y
y= 0
now ,from (1) and (3)
4.0 × 10⁻⁵ = k[0.1]^x [0.1]^y equation (1)
16.0 × 10⁻⁵ = k[0.2]^x [0.1]^y equation (2)
equation (2) / equation (1)
4 = (0.2)^x / (0.1)^y
(2)² = (2)^x
x = 2
therefore ,
Rate = k[A]² [B]⁰
b) Rate = k[A]² [B]⁰
4 × 10⁻⁵ = k[0.1]² [0.1]⁰
4 × 10⁻⁵ = k (0.01)
k = 4 × 10⁻⁵ / 0.01
k = 4 × 10⁻³
Thus, a) The rate law for reaction is Rate = k[A]² [B]⁰
b) the rate constant is k = 4 × 10⁻³
To learn more about Rate law here
https://brainly.com/question/27160357
#SPJ1
A group of a similar search that proform a particular function is called
Answers
Answer:
Explanation: Tissue if I'm not mistaken
A group of a similar cells that perform a particular function is called a tissue.
What is a Tissue?This is the aggregation of cells which have the same type of structure and therefore performs the same type of function through what is referred to as cell specialization.
The organ is also formed from the aggregation of tissues and is necessary so as to ensure that biochemical activities in the body are performed optimally.
Read more about Tissues here https://brainly.com/question/25331705
#SPJ1
Which of the following compounds has the highest boiling point?HClH2SNH3H2O
Answers
The option (d) is correct- H₂O has the highest boiling point.
What is boiling point?
The boiling point of any liquid is reached at this temperature. The temperature at which a liquid's vapor pressure reaches atmospheric pressure is known as the boiling point of the liquid. The liquid turns into a vapor at this temperature.
What is compounds ?
A material created through the chemical bonding of two or more distinct components. Water (H2O), which is a compound of the elements hydrogen and oxygen, and table salt (NaCl), which is a composite of the elements sodium and chloride, are two examples of compounds.
Therefore, option (d) is correct- H₂O has the highest boiling point.
Learn more about boiling point from the given link.
https://brainly.com/question/24675373
#SPJ4
0.175 moles of aluminum nitrate reacted with a sufficient amount of copper sulfate to produce how many moles of copper nitrate
Answers
Copper metal has a mole weight of 0.45 moles. the complementary interaction of copper and aluminum. 0.45 moles of copper nitrate.
Sodium is created when sodium chloride and silver nitrate combine. Next, we must determine how many moles of each reactant there are. This molarity concentration unit connects moles of solute to volume of solution. Chemical processes are expressed in terms of moles of reactants and products. Aluminum oxide is created when aluminum and oxygen react. The lowest set of whole number coefficients in a balanced equation.
to know more about copper nitrate please visit.
https://brainly.com/question/12768349
#SPJ4
Which ink spot had the most pigments? Question 3 options: black red blue green Which solvent caused the ink from the dots to move the most? Question 4 options: half water, half alcohol alcohol vegetable oil water
Answers
Answer:
The water/alcohol solvent caused the dots to move the most. The oil solvent
Explanation:
An organic compound consist the following by mass 0.188g of carbon, 0.062 of H and 0.25g of O. If the vapor density of the compound is 16. Determine the molecular formula
Answers
The compound has the molecular formula CH₄O. H : O = 1 : 4 : 1 As a result, the empirical formula is CH₄O.
Equating empirical formula :The organic compound sample's mass is 0.5 g, or (0.188 + 0.062 + 0.25) The vapor density should not have a unit.
The organic compound has a molar mass = 16 × 2 g/mol
= 32 g/mol
In 1 mol of the compound:Moles of C atoms = (32 g) × (0.188/0.5) / (12 g/mol) = 1
Moles of H atoms = (32 g) × (0.062/0.5) / (1 g/mol) = 4
Moles of O atoms = (32 g) × (0.25/0.5) / (16 g/mol) = 1
Hence, molecular formula = CH₄O
Thus, the compound has the molecular formula CH₄O. H : O = 1 : 4 : 1 As a result, the empirical formula is CH₄O.
What are examples of organic compounds?Organic molecules contain covalently bound carbon and hydrogen as well as frequently additional elements. Benzoic acid, diethyl malonate, propanoic acid, butanoic acid, malonic acid, amines, heterocyclic compounds, and benzoic aldehyde are all examples of organic compounds. Organic substances include the carbohydrates, fats (lipids), proteins, and nucleic acids that make up the components of life. Organic molecules include petroleum and natural gas, which constitute the majority of fossil fuels.
Learn more about organic compounds :
brainly.com/question/5994723
#SPJ1
A hot ballon with mass of 400 kilograms moves across the aky with 3,200 joules of kinetic energy. The velocity of the ballon is
Answers
Answer:
4 m/s
Explanation:
formula is v = (KE/.5m)^1/2
there is a calculator
https://www.calculatorsoup.com/calculators/physics/kinetic.php
Please help!!! Only one try. Explain why the answer is the right answer
Which equation is an example of artificial transmutation

Answers
Answer:Nitrogen can be transformed into oxygen by bombarding an alpha particle into the nucleus of nitrogen. An atom of hydrogen is produced as part of the transformation. Aluminum is transformed into phosphorous by combining the nucleus with an alpha particle
Explanation:
The phases of the moon.
Can be predicted years into the future a
Always happen in the same pattern b
Can occur in ANY order c
a and b are correct d
Answers
Answer:
c. or b._____________
Explanation:
correct me
PLSSSSS I NEED HELP MY AMPLIFY SIM WON’T WORK I DONT KNOW WHAT TO DO FOR AMPLIFY TAB 3.5.!! IM IN THE PURPLE GROUP !! The lead chemist wants you to determine what is happening to the freedom of movement of an object’s molecules when you smell something. Is it possible to smell a chocolate bar when it is a solid? Launch the Sim and investigate.
Use the Sim to determine if the molecules of a substance can be in two different phases at the same time.
Go through each substance and see if you can get it to exist in two phases at once.
Record as much evidence as you can in the table below.
Answers
When a chocolate bar is solid, it is able to smell it. It is also important to note that a substance 's molecules cannot be in two distinct phases at the a time.
Why is this the case ?In general, it is not possible for a the molecules of the chocolate to be in two distinct phases at the same time.
It must be noted that smelling a solid, such as a chocolate bar, however, mean the release of loose molecules from the solid, which may then move through the air and reach our olfactory receptors in our nose, allowing us to sense the fragrance.
Learn more about phase transition:
https://brainly.com/question/29795670
#SPJ1
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced.
reaction
4 HF (9) + SiO₂ (s) → SiF4(9) + 2 H₂O(g)
2 Cl(aq) + 2 H₂O 2OH(aq) + H₂(g) + Cl₂(9)
-
H₂S(aq) + 2NaOH(aq) → Na₂S(aq) + 2 H₂O(
2 H₂(g) + O₂(g) 2 H₂O(g)
-
oxidized
O
O
O
highlighted atom is being...
O
reduced
O
O
O
neither oxidized
nor reduced
O
O
O
S
Answers
- In reactions 1 and 2, the highlighted atom (oxygen) is reduced.
- In reaction 3, the highlighted atom (sulfur) is neither oxidized nor reduced.
- In reaction 4, the highlighted atom (oxygen) is reduced.
In the given chemical reactions, we need to identify whether the highlighted atom is being oxidized or reduced. Let's analyze each reaction individually:
Reaction 1: 4 HF (g) + SiO₂ (s) → SiF₄ (g) + 2 H₂O (g)
In this reaction, the highlighted atom is oxygen (O). Oxygen in SiO₂ undergoes a change in oxidation state from -2 to 0 in SiF₄. Therefore, the highlighted atom (oxygen) is reduced.
Reaction 2: 2 Cl (aq) + 2 H₂O (l) → 2 OH (aq) + H₂ (g) + Cl₂ (g)
In this reaction, the highlighted atom is oxygen (O). Oxygen in H₂O undergoes a change in oxidation state from -2 to -1 in OH. Therefore, the highlighted atom (oxygen) is reduced.
Reaction 3: H₂S (aq) + 2 NaOH (aq) → Na₂S (aq) + 2 H₂O (l)
In this reaction, the highlighted atom is sulfur (S). Sulfur in H₂S undergoes a change in oxidation state from -2 to -2 in Na₂S. Therefore, the highlighted atom (sulfur) is neither oxidized nor reduced.
Reaction 4: 2 H₂ (g) + O₂ (g) → 2 H₂O (g)
In this reaction, the highlighted atom is oxygen (O). Oxygen in O₂ undergoes a change in oxidation state from 0 to -2 in H₂O. Therefore, the highlighted atom (oxygen) is reduced.
To summarize:
- In reactions 1 and 2, the highlighted atom (oxygen) is reduced.
- In reaction 3, the highlighted atom (sulfur) is neither oxidized nor reduced.
- In reaction 4, the highlighted atom (oxygen) is reduced.
It's important to note that oxidation and reduction involve changes in the oxidation state of atoms, indicating the gain or loss of electrons. The analysis above is based on the change in oxidation state of the highlighted atom in each reaction.
for more questions on oxidized
https://brainly.com/question/13182308
#SPJ8