Which statement BEST describes electromagnetic and mechanical waves?
A.
Electromagnetic waves and mechanical waves both do not require a medium.
B.
Mechanical waves require a medium and electromagnetic waves do not.
C.
Electromagnetic waves require a medium and mechanical waves do not.
D.
Mechanical waves and electromagnetic waves both require a medium.
Answers
Answer:
C I believe
Explanation:
Related Questions
Conjugation requires ______ orbitals on three or more adjacent atoms in a structure.
Answers
Conjugation requires p orbitals on three or more adjacent atoms in a structure.
What is conjugation?The overlap of one p orbital with another over an adjacent bond is referred to as conjugation (in transition metals d orbitals can be involved). A conjugated system in a molecule is a system of connected p orbitals with delocalized electrons that reduces the overall energy of the molecule and promotes stability. It is typically represented by alternating single and multiple bonds. The system, which can be cyclic, acyclic, linear, or mixed, may contain lone pairs, radicals, or carbenium ions. Johannes Thiele, a German chemist, created the term "conjugated" in 1899. A conjugated system has a band of overlapping p orbitals that bridges the interjacent places where simple diagrams show no link.
To learn more about conjugation , visit:
https://brainly.com/question/15579188
#SPJ4
Name two properties that make solutions different from colloids.
Answers
Answer:
1. the apperance of a solution is clear while that of a colloid is dull
2. colloids are heterogenou while solutions are homogenous
What amount of hydrogen(H), in moles, is present in the water (H2O) produced

Answers
Explanation:
According to the problem 0.2916 g of water were produced, so we have to find the number of moles of H in that sample of water. First we will convert those grams into moles using the molar mass of water.
molar mass of H = 1.01 g/mol
molar mass of O = 16.00 g/mol
molar mass of H₂O = 2 * 1.01 g/mol + 1 * 16.00 g/mol
molar mass of H₂O = 18.02 g/mol
moles of H₂O = 0.2916 g * 1 mol/(18.02 g)
moles of H₂O = 0.01618 moles
One molecule of H₂O contains two atoms of H. So 1 mol of H₂O molecules will contain 2 moles of H atoms. We can use that relationship to find the answer to our problem.
1 mol of H₂O = 2 moles of H
moles of H = 0.01618 moles of H₂O* 2 moles of H/(1 mol of H₂O)
moles of H = 0.03236 mol
Answer: 0.03236 mol of H atoms are present in the water produced.
Kinetic energy is energy an object has because of its:
Question 1 options:
composition
position
density
motion
Answers
Answer:
I think its Motion
Explanation:
What is produced when a strong base reacts with the bicarbonate buffer system in the human body?
a.) carbon dioxide
b.) bicarbonate ions
c.) carbonic base
d.) water
Answers
Answer:
The answer is Bicarbonate ions.
Answer:
C. Carbonic base
Explanation:
EDG2021
the rate constant for the forward reaction, 1k1 , is 255 l⋅mol−1⋅min−1255 l⋅mol−1⋅min−1 and the rate constant for the reverse reaction, 1k1 , is 391 l⋅mol−1⋅min−1391 l⋅mol−1⋅min−1 at a given
Answers
These rate constants are used to determine the rate of the forward and reverse reactions, respectively, at a given condition.
The rate of the forward reaction can be calculated using the equation: rate of forward reaction = 1k1 [reactants]where [reactants] represents the concentration of the reactants. Similarly, the rate of the reverse reaction can be calculated using the equation:rate of reverse reaction = 1k-1 [products]where [products] represents the concentration of the products. At a given condition, the rate of the forward and reverse reactions may be equal, which is known as the equilibrium state.
At this point, the rate of the forward reaction is equal to the rate of the reverse reaction, and the concentration of the reactants and products remain constant. The equilibrium constant, Keq, can be calculated using the rate constants at equilibrium:Keq = rate of forward reaction / rate of reverse reaction= 1k1 / 1k-1Knowing the equilibrium constant can help us determine the direction in which a reaction will proceed under certain conditions. If the concentration of the reactants is increased, the rate of the forward reaction will increase, leading to a shift in the equilibrium towards the products.
Learn more about forward reaction here:https://brainly.com/question/30064818
#SPJ11
geol 101 how is the half-life of a radioactive parent isotope defined? group of answer choices the time it takes for half of the parent isotope to decay half the time it takes for the parent isotope to completely decay the time it takes the parent isotope to go through half the decay steps necessary to produce a stable daughter isotope half of the average rate of decay of the parent isotope
Answers
The half-life of a radioactive parent isotope is defined as the time it takes for half of the parent isotope to decay.
The half-life of a radioactive parent isotope is defined as the time it takes for half of the parent isotope to decay. It is a measure of the stability of a radioactive isotope and is used to predict how long it will take for half of the radioactive atoms in a sample to decay.
This can be used to estimate the age of a sample or to determine how long a specific isotope will remain dangerous. The half-life of a radioactive isotope is a constant and is specific to that isotope. It is not affected by temperature, pressure, or chemical environment. The half-life of a radioactive isotope can range from fractions of a second to billions of years.
To learn more about half-life visit: https://brainly.com/question/12341489
#SPJ4
Your question seems incomplete, but I assume the question was:
"How is the half-life of a radioactive parent isotope defined? (group of answer choices)
The time it takes for half of the parent isotope to decay.
Half the time it takes for the parent isotope to completely decay.
The time it takes the parent isotope to go through half the decay steps necessary to produce a stable daughter isotope.
Half of the average rate of decay of the parent isotope."
1. What net ionic equation describes the reaction between Pb(NO3)2( aq) and N a2CO3( aq) ?
O 2Na t( aq) + N0, 2( aq) → 2NaNO3( s)
O Pb 2+( aq) + 2Na t( aq) → 2NaPb( s)
O Pb 2+( aq) + CO3( aq) → PBCO3( s)
O 2Na t( aq) + CO3 2( aq) → NacO3( s)
Answers
Answer: C. Pb^2+(aq) + CO3^-2(aq) —> PbCO3(s)
Explanation:
When 38^88Sr decays to 34^84Kr, a(n) ______________ is emitted
Answers
Answer:
The correct answer is - alpha particle and positron.
Explanation:
In this question, it is given that, 38^88Sr decays to 34^84Kr, which means there is an atomic number decrease by 4, 38 to 34, and atomic mass decreases by 4 as well 88 to 84.
A decrease in the atomic mass is possible only when there is an emission of the alpha particle as an alpha particle is made of 2 protons and 2 neutrons. If an atom emits an alpha particle, there is a change in atomic number as it decreases by two, and its mass number decreases by four.
So after the emission of an alpha particle, the new atom would be
38^88Sr=> 36^84X => 34^84Kr
so there is also two positron emission that leads to decrease in atomic number by one with each emission:
38^88Sr=> 2^4He+ 36^84X => 36^84X + 2(1^0β+) => 34^84Kr
Positron decay is the conversion of a proton into a neutron with the emission of a positron that causes the atomic number is decreased by one, which causes a change in the elemental identity of the daughter isotope.
Juan's mother drives 7.25 miles southwest to her favorite shopping mall. A. Average velocity is the total displacement divided by the total time taken. What is the average velocity of Juan's mother's automobile if it arrives at the mall in 20.0 minutes? B. Does the average velocity reflect the how fast Juan's mother was driving at every point in her journey? Explain your answer by comparing the terms average velocity and instantaneous velocity.
Answers
Answer:
See explanation
Explanation:
Average velocity = distance traveled/time
distance traveled = 7.25 miles
Time taken = 20 minutes or 0.33 hours
Average velocity = 7.25 miles/0.33 hours = 21.97 miles per hour
This average velocity does not reflect how fast Juan's mother drove at every point in the journey.
At every point in the journey, Juan's mother had an instantaneous velocity given by the velocity at that given instant divided by time taken up to that point.
The instantaneous velocity gives the velocity at particular instants throughout the journey while the average velocity reflects the average velocity of the entire journey.
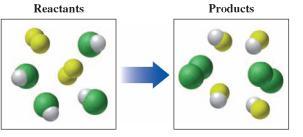
Suppose that green spheres represent chlorine atoms, yellow-green spheres represent fluorine atoms, white spheres represent hydrogen atoms, and all the molecules are gases
Answers
Answer:
a. Reactants: F₂ and HCl molecules
Products: Cl₂ and HF
b. 4HCl + 2F₂ ----> 4HF + 2Cl₂
c. Single replacement reaction
Note: The question is not complete. The complete question is found below as well as in the attachment.
Suppose that green spheres represent chlorine atoms, yellow-green spheres represent fluorine atoms, white spheres represent hydrogen atoms, and all the molecules are gases:
a. write a formula for each of the reactants
b. write a balanced equation for the reaction
c. indicate the type of reaction as combination,decomposition, single replacement, double replacement, or combustion
Explanation:
a. From the models shown in the diagram, The reactants are F₂, HCl while the products are Cl₂ and HF respectively
b. Equation for the reaction is given below;
4HCl + 2F₂ ----> 4HF + 2Cl₂
c. The reaction is single replacement reaction such that fluorine gas molecules replace the chlorine atoms in hydrogen chloride to form hydrogen fluoride with the liberation of chlorine atoms as gaseous molecules.
Name the elements that are found in this equation?
SnO₂+2H₂→Sn+2H₂O
Answers
Answer:
Sn= Tin
Explanation:
Sn= Tin / O= Oxygen / H= hydrogen
i dont know what to do

Answers
Answer:
C
Explanation:
I'm pretty sure it's C sry if it's not
What is true when a reaction has reached equilibrium?
A. The reaction has stopped.
B.The reaction is faster in the forward direction.
C. The reaction rate is equal in both directions.
D.The reaction is faster in the reverse direction.
Answers
A reaction is said to have reached equilibrium when the reaction rate is equal in both directions (c).
Like, take the example of copper in copper sulphate solution. When you add Cu to CuSO4 soln., there won't be any change occurring as the reaction rate is equal (Cu is added to CuSO4 soln., there won't be any change as displacement reaction won't take place due to Cu being the same metal as in CuS04 soln...their reaction is the same).
_____
RainbowSalt2222 ☔
Which type of
chemistry studies
non-carbon based
materials?
A. Analytical Chemistry
B. Organic Chemistry
C. Nuclear Chemistry
D. Inorganic chemistry
Answers
Inorganic chemistry is a type of chemistry studies non-carbon based materials
Organic chemistry is defined as the study of carbon-containing compounds, inorganic chemistry is the study of the remaining means not carbon-containing subset of compounds and inorganic chemistry focuses on structures that do not contain carbon and most commonly these structures include oxygen, silicon, aluminum, iron, calcium, sodium and magnesium and inorganic chemistry also studies synthesis, reactions, structures and properties of compounds
Know more about non carbon
https://brainly.com/question/9477180
#SPJ1
Answer:
in organic chemistry is the type of non carbon based materials.
Explanation: it is a study of behavior and properties of in organic compounds which includes metals, minerals , compounds.
Divergent tectonic plate boundaries most commonly form one: A non-volcanic islands. B ocean trenches C continental mountains D ocean ridges
Answers
Answer:
D. ocean ridges
Explanation:
Answer:
I think A
Explanation:
Most volcanoes form at the boundaries of Earth's tectonic plates. ... The two types of plate boundaries that are most likely to produce volcanic activity are divergent plate boundaries and convergent plate boundaries
What are the Oxidation numbers for H2AsO4-
Answers
i cant understand your question define briefly
What four elements make up the bulk?
Answers
The four elements make up the bulk of all living things are hydrogen, carbon, oxygen and the nitrogen. they make up the human body of about 96 %.
The 25 elements are most important for the life. the four most important are present in about 96 % are :
carbonhydrogenoxygennitrogenthe some of the elements are present in about 3.5 % are : calcium, phosphorus, potassium, sulfur, sodium , chlorine and magnesium . the rest are present in the trace amount about 0.5 %. oxygen is the most common element present in the human body. carbon is versatile element which can make the bond by itself.
To learn more about elements here
https://brainly.com/question/4573241
#SPJ4
200 L of a gas at 10 atm pressure and 400 K is cooled to 100 K and reduced to 2 atm pressure. What is the new volume?
Answers
Answer:
250L
Explanation:
Data;
V1 = 200L
P1 = 10atm
T1 = 400K
T2 = 100K
P2 = 2atm
V2 = ?
To solve this question, we'll have to use the combined gas equation which is a combination of Boyle's law, Charles law and pressure law.
From combined gas equation,
(P1 × V1) / T1 = (P2 × V2) / T2
P1 × V1 × T2 = P2 × V2 × T1
Solve for V2,
V2 = (P1 × V1 × T2) / P2 × T1
V2 = (10 × 200 × 100) / (2 × 400)
V2 = 200,000 / 800
V2 = 250L
The final volume of the gas is 250L
When two oxygen atoms bond together in O2, what type of covalent bond do they form?
A double bond, because they overlap orbitals to share one pair of electrons
A double bond because every overlap orbitals to share two pairs of electrons
Answers
Answer:
Not C
Explanation:
Just took it
Answer:
A double bond, because they overlap orbitals to share two pairs of electrons.
Explanation:
I took a quiz with this question and i got it correct.
Suppose cobalt-60 undergoes a type of radioactive decay that does not
change the identity of the isotope. which type of decay did the isotope
undergo?
a. delta
b. gamma
c. alpha
d. beta
Answers
how many grams of tin (ll) fluoride are produced if 45.0 grams HF are reacted
Answers
Approximately 176.3 grams of tin (II) fluoride (SnF2) are produced when 45.0 grams of HF react.
The balanced chemical equation for the reaction between hydrofluoric acid (HF) and tin (II) fluoride (SnF2) is:
2 HF + SnF2 → SnF4 + 2 HCl
This equation tells us that for every 2 moles of HF that react with SnF2, we will get 1 mole of SnF4 produced.
To determine how many grams of SnF2 are produced from 45.0 grams of HF, we need to first convert the mass of HF to moles using its molar mass. The molar mass of HF is approximately 20.01 g/mol:
45.0 g HF × (1 mol HF / 20.01 g HF) = 2.25 mol HF
According to the balanced equation, 2 moles of HF react with 1 mole of SnF2. Therefore, we can determine the moles of SnF2 produced by dividing the moles of HF by 2:
2.25 mol HF ÷ 2 = 1.125 mol SnF2
Finally, we can convert the moles of SnF2 to grams using its molar mass, which is approximately 156.70 g/mol:
1.125 mol SnF2 × (156.70 g SnF2 / 1 mol SnF2) ≈ 176.3 g SnF2
For more question on tin (II) fluoride click on
https://brainly.com/question/29715194
#SPJ11
A sample of trifluoroacetic acid, C
2
HF
3
O
2
, contains 66.9 g of fluorine. Calculate the mass of the trifluoroacetic acid sample.
Answers
The molar mass of trifluoroacetic acid (C2HF3O2) is 111.5 g/mol. Therefore, the maximum mass of the trifluoroacetic acid sample containing 66.9 g of fluorine can be calculated.
Trifluoroacetic acid, with the chemical formula C2HF3O2, contains 3 fluorine atoms per molecule. Given that the mass of fluorine in the sample is 66.9 g, we can calculate the mass of the entire trifluoroacetic acid sample.
The molar mass of trifluoroacetic acid can be calculated by summing the atomic masses of its constituent elements. The atomic masses of carbon (C), hydrogen (H), fluorine (F), and oxygen (O) are approximately 12.01 g/mol, 1.01 g/mol, 18.99 g/mol, and 16.00 g/mol, respectively.
Molar mass of trifluoroacetic acid:
2 * (molar mass of carbon) + 1 * (molar mass of hydrogen) + 3 * (molar mass of fluorine) + 2 * (molar mass of oxygen)
= 2 * 12.01 g/mol + 1 * 1.01 g/mol + 3 * 18.99 g/mol + 2 * 16.00 g/mol
= 40.03 g/mol
Next, we can calculate the number of moles of fluorine in the sample using its molar mass:
Number of moles of fluorine = Mass of fluorine / molar mass of fluorine
= 66.9 g / 18.99 g/mol
= 3.52 mol
Since there are 3 fluorine atoms in one molecule of trifluoroacetic acid, the number of moles of trifluoroacetic acid is also 3.52 mol.
Finally, we can calculate the mass of the trifluoroacetic acid sample using its molar mass:
Mass of trifluoroacetic acid sample = Number of moles of trifluoroacetic acid * molar mass of trifluoroacetic acid
= 3.52 mol * 40.03 g/mol
= 111.5 g
Therefore, the mass of the trifluoroacetic acid sample is 111.5 g.
Learn more about trifluoroacetic here:
https://brainly.com/question/30846307
#SPJ11
what is the relationship between these numbers: number of atomic orbitals that hybridize, and the number of electron groups around the central atom? group of answer choices the number of atomic orbitals needed is the same as the number of electron groups around a central atom.
Answers
The number of atomic orbitals needed is the same as the number of electron groups around a central atom.
When a central atom forms covalent bonds with other atoms, the electron groups around the central atom determine the number of hybrid orbitals needed to form those bonds. Each electron group, whether it is a lone pair or a bond, requires an atomic orbital to hybridize. Therefore, the number of atomic orbitals needed is directly related to the number of electron groups around the central atom.
In summary, the relationship between the number of atomic orbitals that hybridize and the number of electron groups around the central atom is that they are equal. This relationship is important in understanding the geometry and bonding of molecules.
To know more about atomic orbitals, visit;
https://brainly.com/question/20319149
#SPJ11
a tank of mixed gases, consisting of h2, n2, ar, and h2o, is held at a constant temperature. which gas has the highest average velocity?
Answers
In a tank of mixed gases, consisting of H₂, N₂, Ar, and H₂O, is held at a constant temperature. The gas has the highest average velocity is H₂.
The velocity of all gas particles is inversely proportional to the molecular weight of the gas. Light gases have higher kinetic energy than heavy gases. From the list given, with an atomic mass of 2, N₂ with an atomic mass of 28, Ar with an atomic mass of 40, and H₂O with an atomic mass of 18. H₂ is the lightest of the four gases, and therefore the lightest. Velocity contributes to the kinetic energy of the particles.
Gas particles are always in motion, and all moving objects have kinetic energy (Ek). Molecules in a sample of gas share an average kinetic energy. However, individual molecules exhibit a kinetic energy distribution due to their velocity distribution. This velocity distribution arises from the collisions that occur between molecules in the gas phase. Although these collisions are elastic (no net loss of energy), the individual velocities of each molecule involved in the collision can change.
Kinetic molecule theory states that the average kinetic energy of gas particles is proportional to the absolute temperature of the gas. This can be expressed by the following equation, where k is the Boltzmann constant. The Boltzmann constant is simply the gas constant R divided by the Avogadro constant (NA). Bars above specific terms indicate that these are averages.
Ek = 3/2 (KT)
Since the average kinetic energy is related to both absolute temperature and molecular velocity, the above equation can be combined with the previous one to find the effective velocity.
Ek = 1/2(mu²) = 3/2 (KT)
=√u² =√(3KT/m)
This shows that the RMS velocity is related to temperature. This equation can be manipulated further by multiplying the numerator and denominator by the Avogadro constant (NA) to obtain a form using the gas constant (R) and molar mass (M).
√u² =√(3Rt/m)
The form of this equation shows that the effective velocity of gas molecules is also related to the molar mass of matter. Comparing two gases of different molar masses at the same temperature shows that the gas with the lower molar mass has a higher effective velocity, even though they have the same average kinetic energy.
Learn more about velocity of gas here https://brainly.com/question/12376371
#SPJ4
what is the approximate residence time for a water molecule in a river?
Answers
The residence time for a water molecule in a river is typically between 1 and 5 days.
What is molecule?A molecule is the smallest particle of a substance that can still be identified as that substance. Atoms are held together via molecular bonds to form molecules. Molecules can be as little as two atoms or as large as thousands of atoms. Atoms are held together via molecular bonds to form molecules. Molecules can be as little as two atoms or as large as thousands of atoms. The properties of a molecule are determined by the arrangement of its atoms and the type of chemical bonds that hold them together. Molecules can exist as individual particles or as part of a larger structure. Molecules can be found in all forms of matter including gases, liquids, and solids. They are the building blocks of all living things and play an important role in chemical reactions.
To learn more about molecule
https://brainly.com/question/26044300
#SPJ4
Which group would generally have the lowest first ionization energy?A) Transition MetalsB) Alkali MetalsC) Noble GasesD) Alkaline Earth MetalsE) Halogens
Answers
Alkali metals exhibit the lowest first ionization energy.
Alkali metals are a group of elements. Their standard valence shell setup is ns1. Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Lithium (Li), Hydrogen (H), and Lithium are some of them (Cs).
The alkali metal with the lowest ionization energy is cesium.
What is ionization energy?Ionization energy is the energy required to remove an electron from a neutral atom or a positive ion. It is a measure of the tendency of an atom or ion to lose an electron and become a cation. The ionization energy is usually expressed in units of electron volts (eV) or kilojoules per mole (kJ/mol). The first ionization energy is the energy required to remove the outermost electron from an atom in its ground state, while the second ionization energy is the energy required to remove the second outermost electron, and so on. Generally, the ionization energy increases as we move across a period in the periodic table, and decreases as we move down a group. This is because as we move across a period, the effective nuclear charge increases, which makes it more difficult to remove an electron from the outermost shell.
To learn more about the ionization energy, click the given link ;
brainly.com/question/20658080
#SPJ4
A sample of certain gas have Volume of 1.25 L ATM _125 degree Celsius and5.0 ATM the gas is compressed 50.0 ATM a volume of 325 mL. what is final temperature?
Answers
The final temperature of the gas is approximately 40.96 Kelvin.
To determine the final temperature of the gas, we can use the ideal gas law, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
First, let's convert the given temperatures to Kelvin. We have:
Initial temperature: -125 degrees Celsius = 148 K (approximate)
Final temperature: Unknown
The initial conditions of the gas are as follows:
Initial pressure (P1) = 1.25 atm
Initial volume (V1) = 1250 mL = 1.25 L (since 1 L = 1000 mL)
Initial temperature (T1) = 148 K
The final conditions of the gas are as follows:
Final pressure (P2) = 50.0 atm
Final volume (V2) = 325 mL = 0.325 L
Final temperature (T2) = Unknown
Using the ideal gas law, we can set up the following equation:
(P1 * V1) / T1 = (P2 * V2) / T2
Substituting the known values:
(1.25 atm * 1.25 L) / 148 K = (50.0 atm * 0.325 L) / T2
Simplifying the equation:
T2 = (50.0 atm * 0.325 L * 148 K) / (1.25 atm * 1.25 L)
T2 = 40.96 K
For more such questions on temperature visit;'
https://brainly.com/question/4735135
#SPJ8
what is the impact of the addition of acid to the final texture, appearance, and flavor of the cooked legumes? describe what you observed in your lab experiments
Answers
Acid will make the final texture of the legumes mushy. The flavor of legumes will be sour and the appearance with be flat.
The primary difference among legumes and beans is that the seeds collected from exclusive plant life are called beans, A legume is any plant that bears its fruit internal a pod. Legume is an umbrella time period that consists of beans and pulses. As a consequence, all beans are considered a legume, but not all legumes are considered beans.
Legumes a class of greens that consists of beans, peas and lentils are the various most versatile and nutritious ingredients to be had. Legumes are normally low in fat, include no cholesterol, and are high in folate, potassium, iron and magnesium. In addition they incorporate useful fats and soluble and insoluble fiber.
Learn more about legumes here:- https://brainly.com/question/19900764
#SPJ4
calculate each of the following quantities in 0.160 mol of C6H14O. calculate the number of atoms of H. calculate the number of atoms of C.
Answers
Answer:
To calculate the number of atoms of H and C in 0.160 mol of C6H14O, we need to first determine the number of moles of each element present in C6H14O.
The molecular formula of C6H14O shows that there are 6 carbon atoms, 14 hydrogen atoms, and 1 oxygen atom in each molecule of C6H14O.
The molar mass of C6H14O can be calculated as:
Molar mass of C6H14O = (6 × atomic mass of C) + (14 × atomic mass of H) + (1 × atomic mass of O)
= (6 × 12.01 g/mol) + (14 × 1.01 g/mol) + (1 × 16.00 g/mol)
= 86.18 g/mol
Therefore, 0.160 mol of C6H14O has a mass of:
Mass = molar mass × number of moles
= 86.18 g/mol × 0.160 mol
= 13.79 g
Now we can calculate the number of atoms of H and C in 0.160 mol of C6H14O.
Number of atoms of H:
Number of moles of H = 14 × 0.160 mol = 2.24 mol
Number of atoms of H = 2.24 mol × Avogadro's number
= 2.24 mol × 6.022 × 10^23/mol
= 1.35 × 10^24 atoms of H
Therefore, there are 1.35 × 10^24 atoms of hydrogen in 0.160 mol of C6H14O.
Number of atoms of C:
Number of moles of C = 6 × 0.160 mol = 0.96 mol
Number of atoms of C = 0.96 mol × Avogadro's number
= 0.96 mol × 6.022 × 10^23/mol
= 5.78 × 10^23 atoms of C
Therefore, there are 5.78 × 10^23 atoms of carbon in 0.160 mol of C6H14O.
Explanation: