Answers
Answer:
Atoms are made of protons, neutrons and electrons.
Explanation:
The Dalton's atomic theory was an early attempt at describing the properties of atoms. It stipulated that atoms were the smallest indivisible particle of a substance. Chemical reactions occur as a result of a combination or separation of atoms. Atoms of the same element are exactly alike and differ from atoms of other elements. Atoms can neither be created nor destroyed.
As time went on, modern scientific evidence began to modify the original postulates of the Dalton's atomic theory. It was not postulated in 1805 that atoms were composed of subatomic particles; electrons, neutrons and protons. Dalton's theory held the atom to be 'indivisible'. However in 1897, JJ Thompson discovered the electron. Subsequently, the proton and neutrons were discovered. This shows that the atom in itself consisted of even smaller particles.
Related Questions
A paper airplane is thrown across the room. The distance the paper airplane
traveled is measured. Then, a paper airplane made with a different type of
paper is thrown across the room. The distance it traveled is measured. This is
repeated two more times with two different types of paper.
What question was this experiment trying to answer?
How does the
type of paper of
a paper airplane
affect the
distance it
travels?
What paper is
heavier?
Which paper is
prettier?
None of the
answers
Answers
How does the type of paper of a paper airplane affect the distance it travels?
Explanation:The experiment was performed with different types of paper, all paper have different quality, chemical composition,density & all this properties of paper will affect the distance travelled by the paper airplane. hence the motive of experiment was to check how different paper affect the distance travelled by the paper airplane.
\( \sf \small Thanks \: for \: joining \: brainly \: community!\)
Which of the following are organic compounds?
Select all that apply.
water (H2O)
methane (CH4)
propane (C3H8)
ozone (O3)
Answers
Answer:
Methane
Propane
Explanation:
The compounds that contain C as an essential atom are called organic compounds.
Using this reversible reaction, answer the questions below:
N2O4 ⇔2NO2
(colorless) (reddish-brown)
-As the temperature increased, what happened to the N2O4 concentration?
-Was the formation of reactants or products favored by the addition of heat?
-Which reaction is exothermic? Right to left or left to right?
-If the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct?
N2O4⇔ 2NO2 + 14 kcal
N2O4 ⇔2NO2, HR = +14 kcal
N2O4 + 14 kcal ⇔2NO2
N2O4 ⇔2NO2, HR = -14 kcal

Answers
Answer:
1) As the temperature increased what happened to the N2O4 concentration, it decreased
2) Formation of products, products are the right hand side of the equation.
3) Right to left is exothermic
4) Change in enthalpy N2O4 ⇔2NO2, HR = -14 kcal
As it's an exothermic reaction, the enthalpy of the reactants is higher than the products and the sign of the HR will be negative.
Explanation:
N2O4 ⇔2NO2
(colorless) (reddish-brown)
1) As the temperature increased what happened to the N2O4 concentration, it decreased
2) Formation of products, products are the right hand side of the equation.
3) Right to left is exothermic
4) Change in enthalpy N2O4 ⇔2NO2, HR = -14 kcal
As it's an exothermic reaction, the enthalpy of the reactants is higher than the products and the sign of the HR will be negative.
Please read the question and choose the correct answer. Thank you.

Answers
The pH of the solution represent in the diagram, given that the solution contains 1 mole of H⁺ is 2 L is 0.3 (option B)
How do i determine the pH of the solution?We'll begin by obtaining the hydrogen ion, H⁺ concentration in the solution. This is shown below:
Mole of H⁺ = 1 moleVolume = 2 LHydrogen ion, H⁺ concentration = ?Concentration = mole / volume
Hydrogen ion, H⁺ concentration = 1 / 2
Hydrogen ion, H⁺ concentration = 0.5 M
Finally, we shall determine the pH of the solution. Details below:
Hydrogen ion concentration [H⁺] = 0.5 MpH of solution = ?pH = -Log [H⁺]
pH = -Log 0.5
pH = 0.3
Thus, we can conclude that the pH of the solution is 0.3 (option B)
Learn more about pH:
https://brainly.com/question/22983829
#SPJ1
Which statement is one of the assumptions of Kinetic Molecular Theory regarding gasses?
A Collisions between gas particles are inelastic.
The temperature of the gas depends on the average potential energy of the gas particles.
Gas particles are much larger than the distance between them.
The volume of a gas sample mostly consists of empty space between the moving gas particles.
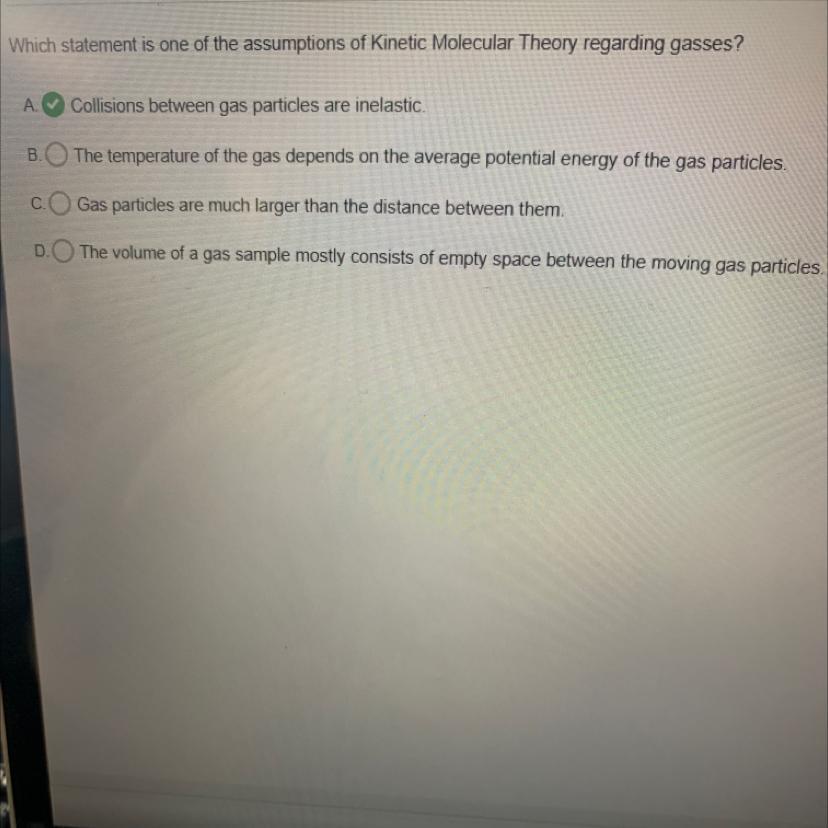
Answers
The statement that "The volume of a gas sample mostly consists of empty space between the moving gas particles" is one of the assumptions of the Kinetic Molecular Theory regarding gases.
What is Collision?
There are different types of collisions, depending on the nature of the objects involved, the speed and direction of their motion, and the type of contact that occurs. For example, elastic collisions are those in which the total kinetic energy of the colliding objects is conserved, meaning that no energy is lost or gained during the collision. In contrast, inelastic collisions are those in which some of the kinetic energy is transformed into other forms of energy, such as heat or sound.
The Kinetic Molecular Theory is a model that describes the behavior of gases. One of the main assumptions of this theory is that gas particles are in constant random motion and move in a straight line until they collide with other particles or the walls of their container.
Another important assumption of this theory is that the volume of a gas sample mostly consists of empty space between the moving gas particles. This means that gas particles are assumed to be very small compared to the overall volume of the gas sample. Therefore, the particles do not occupy all of the available space in the container, but instead only occupy a small portion of it.
Learn more about Collision from given link
https://brainly.com/question/24915434
#SPJ1
Write the unbalanced chemical
equation for the following reaction:
butane (C4H10) + oxygen gas yields
carbon dioxide + water

Answers
Answer:
C₄H₁₀ + O₂ --> CO₂ + H₂O
Explanation:
The other reactant is stated below the reaction, which is oxygen.
Oxygen is a diatomic element meaning there are usually 2 bonded oxygens. O is incorrect since oxygen is never found as a single atom.
The products are stated below the question. The products are:
- Carbon dioxide
- Water
The chemical formula for carbon dioxide is CO₂
The chemical formula for water is H₂O
Both of the above formulas are very common and are widely known.
I don’t have baking powder or all of the 10 subs I found online. I wanna make pancakes any suggestions
Answers
Answer:
flour is the main ingredient sooo you need flour,sugar,salt milk,eggs,vegetable oil,vanilla optional.you can add baking powder or baking soda optional.
Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.)
Note: you are writing the molecular, and not the net ionic equation.
NaClO3(aq) + H2O(l)
Answers
Answer:
HClO₃(aq) + NaOH(aq) ⇒ NaClO₃(aq) + H₂O(l)
Explanation:
We have the products of a reaction and we have to predict the reactants. Since the products are salt and water, this must be a neutralization reaction. In a neutralization reaction, an acid reacts with a base. To form NaClO₃, the acid must be HClO₃(aq) and the base NaOH(aq). The balanced chemical equation is:
HClO₃(aq) + NaOH(aq) ⇒ NaClO₃(aq) + H₂O(l)
3.Classify each reaction as exothermic or endothermic. (4 marks) a.Energy + SO2(g) S(g) + O2(g) _________________________________b.Draw a potential energy diagram for the reaction above:
Answers
Answer:
Explanation:
a) Here, we want to get the class of the reaction
An endothermic reaction absorbs energy from the surroundings and as such, the change in enthalpy value is positive
This can be deduced from the position of energy in the chemical equation. Since it is added to the reactant side, it is for an endothermic reaction
b) Here, we want to draw a potential energy diagram for the reaction
We have this as follows:

Which is a characteristic of an aqueous solution of HNO3?
A.
It conducts electricity.
B.
It forms OH-ions.
C.
It turns litmus blue.
D.
It turns phenolphthalein pink.
Answers
Answer:
A. it conducts electricity
Explanation:
A. Since Nitric Acid is a strong acid, which means it is a strong oxidizing agent, this makes Nitric Acid a good conductor of electricity.
B. Basic solutions are only going to form OH⁻ ions. A Nitric Acid solution will form H⁺ ions.
C. Alkaline substances (pH greater than 7) are more likely to turn blue, so HNO₃ would turn that blue into a red color.
D. I don't know much about phenolphthalein , all I know is that is is pink in Alkaline solutions (basic) and colorless in acidic solutions.
I hope this helps, good luck!
The standard free energy that is required for the sodium-potassium ATPase to pump two K ions into the cell and three Na ions is 43.8 kJ/mol but the standard free energy change of hydrolysis of ATP is only -32 kJ/mol. This apparent imbalance of free energy can be accounted for because ________.
Answers
Answer:
Explanation:
This apparent disparity of the free energy can be taken into account because:
the free energy produced by the hydrolysis of one ATP is adequate enough under psychological circumstances.
The Na-K ATPase aids the pumping of Na+ ions out of the cell and K+ ions into the cell. These actions occurring against their potential(concentration) gradients, which may be produced by hydrolyzing one ATP molecule.
Determine the
Cl
for NeMut1:Wt using the data presented in part 2 of the case study.
1×10 −10
1×10 10
1×10 −7
1×10 7
Cl
cannot be calculated from the data given 4. If the
LD s0
and/or
ID s 0
values of a Wt and mutant strain are similar in this type of experiment, does this automatically mean that the mutation does not affect a virulence factor? Why or why not? Part B. The researchers decided to determine the
Cl
of each of the mutants, again using the horse infection model. The results are summarized in the table below: 5. Determine the
CI
for NeMutl:Wt and NeMut2:Wt. 6. Interpret your results from question 5 above.
Answers
To determine the Cl for NeMutl:Wt, you need to use the data from part 2 of the case study. The data is given as 1x10-10 for the Wt strain and 1x10-7 for the mutant strain. To calculate the Cl, we use the following equation: Cl = 1/[(1/ID50) - (1/LD50)]. Using this equation, we can calculate the Cl to be 3x10-3.
To determine the Cl for NeMut2:Wt, we can use the same equation. Using the data from the table in part B, the Cl for NeMut2:Wt can be calculated to be 8x10-3.Interpreting these results, we can see that NeMut1:Wt has a Cl that is roughly 3 times lower than that of NeMut2:Wt. This suggests that the mutation of NeMut1 is significantly affecting a virulence factor, while NeMut2 may not be affecting a virulence factor as significantly.
It is important to note that similar LD50 and/or ID50 values of a Wt and mutant strain does not necessarily mean that the mutation does not affect a virulence factor. This is because the LD50 and ID50 values are used to measure how much of the pathogen is needed to produce a certain effect, but other aspects of the pathogen such as the speed or rate of infection or the amount of toxin produced can still be different and affect the virulence of the strain.
Cl for NeMut1:Wt cannot be calculated from the data presented in part 2 of the case study. The given results are:| Inoculum (LD50) | Mortality (LD50) | CFU/ml of blood | Wild-type | 6.5 × 10−7 | 6.5 × 10−7 | 7.0 × 103 | NeMut1 | 1.0 × 10−10 | 6.5 × 10−7 | 3.0 × 105 | NeMut2 | 2.0 × 10−7 | 2.0 × 10−7 | 2.2 × 103 |Since the Cl cannot be calculated from the data given, the correct option is (d) Cl cannot be calculated from the data given.If the LDs0 and/or IDs0 values of a Wt and mutant strain are similar in this type of experiment, it does not necessarily mean that the mutation does not affect a virulence factor.
This is because mutations can affect different aspects of virulence, and the specific virulence factor being measured may not be impacted by the mutation.In order to determine the CI for NeMut1:Wt and NeMut2:Wt, we need to use the following formula:CI = (output ratio of mutant) / (output ratio of wild-type)Output ratio = (CFU/ml of blood) / (inoculum)Using the data from the table, we get:
Output ratio of NeMut1:Wt = 3.0 × 105 / 1.0 × 10−10 = 3.0 × 1015Output ratio of wild-type = 7.0 × 103 / 6.5 × 10−7 = 1.1 × 1010CI of NeMut1:Wt = (3.0 × 1015) / (1.1 × 1010) = 2.7 × 105Output ratio of NeMut2:Wt = 2.2 × 103 / 2.0 × 10−7 = 1.1 × 1010CI of NeMut2:Wt = (1.1 × 1010) / (1.1 × 1010) = 1Interpretation of results from question 5 above: The CI of NeMut1:Wt is much greater than 1, indicating that NeMut1 is more virulent than the wild-type strain. The CI of NeMut2:Wt is equal to 1, indicating that NeMut2 does not exhibit any significant difference in virulence compared to the wild-type strain.
For more such questions on mutant
https://brainly.com/question/17031191
#SPJ11
5. Consider the diprotic acid H2A with K1=1.00x104 and K2=1.00x108. Find the pH and concentrations of H2A, HA, and A-2 in 0.20 M of the acid solution.
Answers
In a 0.20 M solution of H2A with \(K_1=1.00*10^4\) and \(K_2=1.00*10^8\), the pH is 1.88, and the concentrations of \(H_2A\),\(HA^-\), and\(A^2-\) are approximately \(0.197 M, 2.54*10^{-3} M\), and \(1.95*10^{-15} M\).
To solve this problem, we need to use expressions for the equilibrium concentrations of the species in a diprotic acid:
\([H_2A] = [H_2A]0/(1 + K_1/[H+] + K_1K_2/[H+]^2) \\\)
\([HA^-] = K_1[H_2A]/(1 + K_1/[H+] + K_1K_2/[H+]^2) \\\)
\([A^2-] = K_1K_2[H_2A]/(1 + K_1/[H+] + K_1K_2/[H+]^2)\)
Given \(K_1\) and \(K_2\) values, we can assume that \(K_2\) is much larger than \(K_1\), and thus we can approximate the concentration of \(H_2A\) to be equal to its initial concentration. Therefore, we can simplify the above expressions to:
\([H_2A]\) ≈\([H_2A]0/(1 + K_1/[H+])\)
[HA^-] ≈ \(K_1[H_2A]/(1 + K_1/[H+])\)
\([A^2-]\)≈\(K_1K_2[H_2A]/[H+]^2\)
Substituting the given values:
\([H_2A]\) ≈\(0.20 M/(1 + 1.00*10^4/[H+])\)
[HA^-] ≈ \(1.00*10^4 * 0.20 M/[H+]/(1 + 1.00*10^4/[H+])\)
\([A^2-]\)≈ \(1.00*10^8 * 0.20 M/[H+]^2\)
To find the pH, we can use the equation:
\(pH = -log[H+]\)
After solving the problem iteratively, we get:
pH ≈ 1.88
\([H_2A]\)≈ 0.197 M
[HA^-] ≈ \(2.54*10^{-3} M\)
\([A^2-]\) ≈ \(1.95*10^{-15} M\)
To know more about equilibrium concentrations, here
brainly.com/question/13043707
#SPJ1
--The complete Question is, Consider the diprotic acid H2A with K1=1.00x10^4 and K2=1.00x10^8. If you have a 0.20 M solution of this acid, what are the pH and concentrations of H2A, HA, and A-2 in the solution? --
Question 4 (1 point)
Neon gas is often used to make signs for restaurants and businesses. However, neon only accounts
for 0.0018% of Earth's atmosphere. Scientists are looking for other elements that have similar
properties as neon that could be used as a substitute. Which of the following elements would most
likely be a good substitute?
Oь
Argon
Flourine
Chlorine
Oxygen
d
Submit
Submit

Answers
Answer:
Argon
Explanation:
The element that would most likely be a good substitute for neon is "argon".
These two elements belong to the noble gases in Group 18(8A) of the Periodic Table. They are inert gases - they do not react chemically with other substances.
Argon, as an inert gas is often used whenever an inert atmosphere is needed. Argon is used in incandescent light bulbs. It is used to stop oxygen from corroding the filament. It is also used by welders to protect weld area.
Argon is a good substitute for neon in this context because they possess some physical properties which are similar.
dry cleaners use tetrachloroethylene (C2CL4) to dissolve oil and grease because C2CL4 is

Answers
Answer: Tetrachloroethylene is an nonpolar molecule.
HELPPP ME PLEASEEEEE

Answers
Answer:
a ). Is Acetic Acid
Am not sure about b tho
Write the chemical formula for the following structure. You can't write subscripts so just
do full size subscript numbers.

Answers
Answer:
H2O2
Explanation:
By numbering the atoms we know about that it’s Hydrogen Peroxide
What are Hydrogen Bonds?
A. An extra strong form of the Van der Waals force between molecules
B. The bonds between a hydrogen atom and another atom in a molecule
C. Extra-strong intermolecular attractions between polar molecules
D. The bonds between hydrogen atoms in an H2 molecule
Answers
B. The bonds between a hydrogen atom and another atom in a molecule
Answer: C
Explanation: I got it correct !
Write the abbreviated electron configuration for Al2+.
Answers
The abbreviated electron configuration for Al2+ is [Ne]3s2 3p1
since Al has 13 electrons : [Ne]3s2 3p1
Al2+ means it has lost 2 electrons to become : [Ne]3s1
what is electron configuration?Electronic configurations in chemistry can be describe as the way each electron move independently in an orbital, in an average field created by all other orbitals. which also means the arrangement of electrons in orbitals around an atomic nucleus.
what is an electron?An electron in simple term is a negatively charged subatomic particle that binds together with protons and neutrons to form an atom's nucleus. it can also be seen as a tiny particle of matter that is smaller than an atom and has a negative electrical charge in it.
learn more about electron configuration here:
brainly.com/question/26084288
#SPJ1
1.45g of naphthalene (C10H8) is dissolved in 21.6g of benzene. Calculate the freezing point of the solution given that the freezing point of pure benzene is 5.5°C, and the molal freezing point depression constant is 4.45°C/m
Answers
Therefore, the freezing point of the solution is 3.173°C.
What is freezing point?Freezing point is the temperature at which a liquid substance turns into a solid at a given pressure. At the freezing point, the solid and liquid states of the substance are in equilibrium, meaning that the rate of melting equals the rate of freezing. The freezing point of a pure substance is a characteristic physical property that depends on the substance's chemical composition and can be used to identify it. When a solute is dissolved in a solvent, the freezing point of the solvent is lowered, and the degree of lowering depends on the amount and identity of the solute.
Here,
To solve for the freezing point of the solution, we need to use the formula:
ΔTf = Kf x molality
where:
ΔTf = change in freezing point
Kf = molal freezing point depression constant
molality = moles of solute per kilogram of solvent
First, we need to calculate the molality of the solution:
Mass of naphthalene = 1.45 g
Molar mass of naphthalene = 128.17 g/mol
Number of moles of naphthalene = 1.45 g / 128.17 g/mol = 0.0113 mol
Mass of benzene = 21.6 g
Density of benzene = 0.879 g/mL
Volume of benzene = 21.6 g / 0.879 g/mL = 24.6 mL = 0.0246 L
Mass of benzene = volume x density = 0.0246 L x 0.879 g/mL = 0.0216 kg
molality = moles of solute / mass of solvent (in kg)
molality = 0.0113 mol / 0.0216 kg = 0.523 mol/kg
Now, we can substitute the given values into the formula to solve for ΔTf:
ΔTf = Kf x molality
ΔTf = 4.45°C/m x 0.523 mol/kg
ΔTf = 2.327°C
Finally, we can calculate the freezing point of the solution:
Freezing point of solution = freezing point of solvent - ΔTf
Freezing point of solution = 5.5°C - 2.327°C
Freezing point of solution = 3.173°C
To know more about freezing point,
https://brainly.com/question/2292439
#SPJ1
1. What is the kinetic energy of a cow that has a mass of 1500 kg and is moving at 4
meters per second?
Answers
Answer:
12000j
mass=1500kg
velocity=4m/s
Kinetic Energy (K E) = 1/2 mv^2
=1/2*1500(4^2)
=1*750*16
=12000j
Why do the balls of the Newton’s cradle eventually stop?
Answers
The balls of the Newton’s cradle eventually stop because they loose energy as they collide.
The balls of the Newton’s cradle lose energy to the air as they move through it due to friction.
These balls make sound when they collide, and consequently the balls loose energy to heat upon collision.
The balls loose energy as they collide with each other and eventually stop.
Thus, we can conclude that the balls of the Newton’s cradle eventually stop because they loose energy as they collide.
Learn more about Newton’s cradle here: https://brainly.com/question/14063949
What should be included when designing a scientific question
Answers
When designing a scientific question, you should ensure the question is:
AnswerableSpecificUnderstandableMeasurableWhat is a scientific question?A scientific question is an inquiry that scientists examine via methods such as observation, experimentation or data collection leading to an answer.
They often require specific parameters for setting up experiments along with means for measuring outcomes or phenomenon under investigation while also needing testable results to establish validity. As such successful ones must possess traits like precision in defining boundaries within which observations will take place making them measurable so they may produce documented evidence supporting the validity of their findings
Learn about scientific question here https://brainly.com/question/1675602
#SPJ1
2ca + o2 - 2cao identify the oxidizing and reducing agents
Answers
Answer:
Ca is a reducing agent and O is the oxidizing agent.
Explanation:
calcium is the reducing agent as it reduces oxygen while it oxidize itself and oxygen is an oxidising agent because it oxidized others and reduces itself. in this equation oxygen is reducing and Calcium is oxidising. and as it is stated in the definition that oxidizing agent is the agent which oxidises others and reduces itself and it is also stated that reducing agent reduces others and oxidize itself. so based on this statement calcium is reducing agent and O is a oxidizing agent.
i need all these im not in the mood for it toddayyy


Answers
Answer:
Me too
Explanation:
7. List one example from your daily life (not doing your chemistry homework) in which you converted between two different units. Explain why the conversion was needed.
Answers
Answer:
when my friend weighs himself in stone and I do it in kg.
Water (2470 g ) is heated until it just begins to boil. If the water absorbs 5.47x105 J of heat in the process,
what was the initial temperature of the water?
Express your answer with the appropriate units.
Answers
Answer:
47.01 °C
Explanation:
Q = mcΔt
Mass of water = 2470g
c or specific heat capacity of water = 4.18 J °C g
Q = 5.47 x 10⁵ J, which is expanded 547,000 J
No idea what Δt is, but we do know the water began to boil, and we know that water's boiling point is 100°C, so the final temperature had to be 100°C.
547,000 J = (2470) x (4.18) x (Δt)
Rearrange equation for Δt
(547,000) / (2470 x 4.18) = Δt
Δt = 52.98°C
But we're not done. We're trying to find the INITIAL TEMPERATURE of the water, not the TEMPERATURE CHANGE. We already have the final temperature, which is 100°C, and now we have how much the initial temperature rose by to get to 100°C.
All that we have to do now is 100 - 52.98 = 47.02°C
The initial temperature of the water is 47.02°C
What is the molar mass of Al(BrO2)
Answers
Answer:
The molar mass is 138.8843 g/mol
A major component of gasoline is octane C8H18. When liquid octane is burned in air it reacts with oxygen O2 gas to produce carbon dioxide gas and water vapor. Calculate the moles of water produced by the reaction of 0.10mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Answers
0.072moles water produced by the reaction of 0.10mol of oxygen. In reality, it's one of the International System of Units' seven foundation units (SI).
What is mole?A mole is just a measuring scale. In reality, it's one of the International System of Units' seven foundation units (SI). When already-existing units are insufficient, new ones are created.
The levels at which chemical reactions frequently occur exclude the use of grams, yet utilizing actual numbers of atoms, molecules, or ions would also be unclear. To fill this gap between extremely small and extremely huge numbers, scientists created the mole.
2C\(_8\) H\(_{18}\)+ 25O\(_2\)→ 16CO\(_2\) + 18H\(_2\)O + Heat Energy
0.10mol =oxygen
the mole ratio between oxygen and water is 25:18
moles of water =( 18/25)×0.10=0.072moles
Therefore, 0.072moles water produced by the reaction of 0.10mol of oxygen.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ1
How many atoms are in 2Ca (OH)2
Answers
Answer:
the Ans is 2
Explanation:
marks as BRAINLY
In calcium hydroxide, there are total 6 atoms are present. 2 calcium atoms, 2 oxygen atoms and 2 hydrogen atoms are present in calcium hydroxide molecule.
What is calcium hydroxide ?An inorganic substance having the chemical formula Ca(OH)2 is calcium hydroxide. It is created when quicklime is combined or slaked with water and is a colorless crystal or white powder. It goes by a variety of names, including pickled lime, hydrated lime, caustic lime, builders' lime, and slaked lime.
A white powder without any smell is calcium hydroxide. It is applied in commercial contexts such sewage treatment, paper manufacturing, building, and food processing. It can be used in dentistry and medicine. In root canal fillings, for instance, calcium hydroxide is frequently used.
Calcium oxide is converted to calcium hydroxide, sometimes known as slaked lime, or Ca(OH)2. A little percentage of it dissolves when combined with water, creating a substance known as lime water.
Thus, In calcium hydroxide, there are total 6 atoms are present.
To learn more about calcium hydroxide, follow the link;
https://brainly.com/question/9584549
#SPJ2