Which of the following phase changes involves the transfer of heat from the surroundings to the system?
A
CH4(g)→CH4(l)CH4(g)→CH4(l), because CH4CH4 molecules in the gas phase must absorb energy in order to move closer together, thereby increasing the intermolecular attractions in the solid state.
B
CO2(g)→CO2(s)CO2(g)→CO2(s), because CO2CO2 molecules in the gas phase must absorb energy in order to move closer together, thereby increasing the intermolecular attractions in the liquid state.
C
H2O(l)→H2O(s)H2O(l)→H2O(s), because H2OH2O molecules in the liquid phase must absorb energy in order to create a crystalline structure with strong intermolecular attractions in the solid state.
D
NH3(l)→NH3(g)NH3(l)→NH3(g), because NH3NH3 molecules in the liquid phase must absorb energy in order to overcome their intermolecular attractions and become free gas molecules.
Answers
Because NH3 molecules in the liquid phase require energy to overcome their intermolecular interactions and transform into free gas molecules, this expression reads NH3(l)— NH3(g). Heat is transferred from the environment to the system during phase shifts.
Ammonia has the chemical formula NH3 and is a colourless gas.Nitrogen and hydrogen make up its composition. Its name in aqueous form is ammonium hydroxide. This inorganic substance smells strongly. It is hazardous and corrosive in its concentrated form. Ammonia has a density of 0.769 kg/m3 at STP, making it lighter than air. It is frequently employed as fertiliser. Additionally, it is employed in the production of explosives like TNT and nitrocelluloseAdditionally, it is employed in the manufacture of soda ash and the Ostwald process to produce nitric acid. A strong alkali, such as sodium hydroxide or calcium hydroxide 2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3(g) is heated with an ammonium salt, such as ammonium chloride NH4Cl, to produce ammonia (g)
learn more about ammonium hydroxide Refer: brainly.com/question/12216996
#SPJ4
Related Questions
Which is the equation for a machine's efficiency? (1 point)
O Efficiency=input energy
O Efficiency=
input energy
output energy
output energy
input energy
-
-
output energy
100
O Efficiency
O Efficiency=input energy + output energy
100

Answers
The efficiency of the machine can be obtained from the expression;
Work output/ work input * 100/1
What is the efficiency?The term efficiency has to do with the way in which energy is converted to a good use in the machine. Let us note that a machine is efficient when most of the work done by the machine has been put to a good use. This is shown by a very high value of the efficiency.
Let us note that a machine can only be able to do a useful work when the efficiency of the machine is high and the machine is able to do quite a whole lot of useful work as we shall see below.
As such, we define the efficiency as the ratio of the work input to the the work output multiplied by a hundred. We are now trying to obtain the equation that can more aptly be said to be the efficiency of the machine.
earn more about the efficiency of a machine:https://brainly.com/question/11752408
#SPJ1
What limitations occurs for chalk in vinegar chemistry pd lab experiment?
Also the precautions to take
Need this asap!!
Answers
Answer:
When conducting a chemistry lab experiment using chalk (calcium carbonate) in vinegar (acetic acid), there are several limitations and precautions to be aware of:
Limitations of chalk in vinegar chemistry experiment:
Reaction rate: The reaction between chalk and vinegar is relatively slow, which may require a longer observation period or higher concentration of vinegar to observe significant changes within a reasonable time frame.
Solubility: Chalk may not dissolve completely in vinegar, resulting in incomplete reaction or difficulty in obtaining accurate results.
Product formation: The reaction between chalk and vinegar produces carbon dioxide gas, water, and calcium acetate. The carbon dioxide gas may escape into the atmosphere, leading to loss of product and inaccurate measurements.
pH: Chalk is a basic substance, and the reaction with vinegar, which is acidic, may result in neutralization, leading to a decrease in the overall acidity of the reaction mixture.
Precautions to take in chalk in vinegar chemistry experiment:
Ventilation: The reaction between chalk and vinegar produces carbon dioxide gas, which can displace air and potentially cause asphyxiation in a closed or poorly ventilated area. Conduct the experiment in a well-ventilated area or under a fume hood to ensure adequate air circulation.
Eye and skin protection: Vinegar is an acid and can cause skin and eye irritation. Wear appropriate personal protective equipment (PPE), such as gloves and goggles, to protect yourself from contact with vinegar or any other chemicals used in the experiment.
Chemical handling: Handle the chemicals, including chalk and vinegar, with care, following proper lab safety protocols. Avoid ingestion, inhalation, or direct contact with the chemicals, and dispose of them properly according to local regulations.
Accuracy in measurements: Use calibrated and accurate measuring tools, such as graduated cylinders or burettes, to measure the amount of chalk, vinegar, and other reagents accurately. This will ensure the reliability and accuracy of the experimental results.
Observations: Make careful and detailed observations during the experiment, noting any changes in appearance, gas evolution, or other relevant observations. Take measurements at appropriate intervals and record the data accurately for analysis and interpretation.
It is important to follow good laboratory practices, including proper chemical handling, accurate measurements, and cautious observations, to ensure safe and reliable results in a chalk in vinegar chemistry lab experiment. Consult with a qualified instructor or supervisor for specific guidelines and precautions related to your experiment.
How many moles of NaCl are contained in 0.1 L of a 0.20 M solution?
Answers
Remember that M=mol/liter. We can use this concentration to find the number of moles in 0.1L of solution:
\(0.1L\cdot\frac{0.20mol}{L}=0.02mol\)There are 0.02 moles of NaCl.
2.62 Predict the chemical formulas of the compounds formed by the following pairs of ions: (a) Cr3+ and Br, (b) Fe3+ and O2, (c) Hg22+ and CO2, (d) Ca2+ and CIO3, (e) NH4+ and PO³
Answers
Answer:
(a) Cr3+ and Br- will form CrBr3 (chromium(III) bromide)
(b) Fe3+ and O2- will form Fe2O3 (iron(III) oxide)
(c) Hg22+ and CO32- will form Hg2CO3 (mercury(I) carbonate)
(d) Ca2+ and ClO3- will form Ca(ClO3)2 (calcium chlorate)
(e) NH4+ and PO43- will form (NH4)3PO4 (ammonium phosphate)
Explanation:
chatGPT
The chemical formulas for the compounds formed by the given pairs of ions are: (a) CrBr3, (b) Fe2O3, (c) Hg2(CO3)2, (d) Ca(ClO3)2, and (e) (NH4)3PO4.
Explanation:(a) Cr3+ and Br- : In order to form a neutral compound, the charges of the ions must balance. The charge of Cr3+ is 3+ and the charge of Br- is 1-. To balance the charges, we need three Br- ions for every Cr3+ ion. Therefore, the chemical formula is CrBr3.
(b) Fe3+ and O2- : The charge of Fe3+ is 3+ and the charge of O2- is 2-. To balance the charges, we need two O2- ions for every Fe3+ ion. Therefore, the chemical formula is Fe2O3.
(c) Hg22+ and CO2- : The charge of Hg22+ is 2+ and the charge of CO2- is 2-. The charges are already balanced, so no extra ions are needed. Therefore, the chemical formula is Hg2(CO3)2.
(d) Ca2+ and ClO3- : The charge of Ca2+ is 2+ and the charge of ClO3- is 1-. To balance the charges, we need two ClO3- ions for every Ca2+ ion. Therefore, the chemical formula is Ca(ClO3)2.
(e) NH4+ and PO3- : The charge of NH4+ is 1+ and the charge of PO3- is 3-. To balance the charges, we need three NH4+ ions for every PO3- ion. Therefore, the chemical formula is (NH4)3PO4.
Learn more about Chemical Formulas here:https://brainly.com/question/36379566
#SPJ3
2. Which state of matter is characterized by particles that are close to each other but are not arranged in a definite pattern?
A)liquid
B)plasma
C)solid
D)gas
Answers
Answer:
Solid
Explanation:
Cus its solid, take a brick for example. It's hard and has no space unlike liquid or gas.
27 Which of these would be best to use to separate small iron filings from a mixture with sand?
A a magnet
B filter paper
C a hot plate
D running water
Answers
Option A, a magnet, is the best choice for separating small iron filings from a mixture with sand.
The best option to separate small iron filings from a mixture with sand would be:
A) a magnet
Using a magnet is an effective method to separate iron filings from a mixture. Iron filings are magnetic, while sand is not. By placing a magnet near the mixture, the iron filings will be attracted to the magnet and can be easily separated from the sand. This process is known as magnetic separation.
To separate the iron filings, you can move the magnet over the mixture, and the iron filings will cling to the magnet. This can be repeated until all the iron filings have been separated from the sand.
Filter paper (option B) is not suitable for this purpose because it is primarily used for separating solid particles from a liquid or gas by filtration. In this case, we are dealing with a solid mixture, not a liquid or gas.
A hot plate (option C) is used for heating substances and would not be effective for separating iron filings from sand.
Running water (option D) may help in some cases to separate different components of a mixture, but in this specific scenario, where we want to separate iron filings from sand, using a magnet would be a more efficient and precise method.
In conclusion, option A, a magnet, is the best choice for separating small iron filings from a mixture with sand.
for more such question on mixture visit
https://brainly.com/question/24647756
#SPJ8
a jaguar can run up to 50 miles per hour, how many feet can he run per second? give your answer in scientific notation to one and three significant figures.
Answers
Answer:
50x10^0
Explanation:
How do ions form, and how does the octet rule determine if an atom becomes a cation or an anion?
Answers
When an atom gains or loses electrons from its outermost energy shell, ions form. When an electron or electrons are lost to achieve a full duplet or octet, elements form cations. An anion is formed when an element gains an electron or more than one electron.
What do you mean by octet rule ?The octet rule refers to atoms' preference for having eight electrons in their valence shell. Atoms with fewer than eight electrons are more likely to react and form more stable compounds.
Atoms can satisfy the octet rule in one of two ways. One method is for them to share their valence electrons with other atoms. The second method is to move valence electrons from one atom to another.
Thus, When an atom gains or loses electrons from its outermost energy shell, ions form.
To learn more about the octet rule, follow the link;
https://brainly.com/question/865531
#SPJ1
Write the balanced molecular chemical equation for the reaction in aqueous solution for sodium hydroxide and tin (IV) acetate. If no reaction occurs, simply write only NR.
Be sure to include the proper phases for all species within the reaction.
Answers
The balanced molecular chemical equation for the reaction in aqueous solution for sodium hydroxide and tin (IV) acetate is 4 NaOH (aq) + Sn (CH\(_3\)COO) (aq) → Sn (OH)(s) + 4 CH\(_3\)COONa (aq) .
An equation per a chemical reaction is said to be balanced if both the reactants plus the products have the same number of atoms and total charge for each component of the reaction. In other words, each component of the reaction have an equal balance of mass and charge. The balanced molecular chemical equation for the reaction in aqueous solution for sodium hydroxide and tin (IV) acetate is 4 NaOH (aq) + Sn (CH\(_3\)COO) (aq) → Sn (OH)(s) + 4 CH\(_3\)COONa (aq) .
To know more about balanced equation, here:
https://brainly.com/question/7181548
#SPJ1
What is deltaG for a reaction where
DeltaG = 3.2 kJ/mol and Q = 3.3 at
295 K? (R = 8.314 J/mol K)
Answers
The free energy is 6.128 kJ/mol
What is free energy?Free energy, also known as Gibbs free energy, is a thermodynamic quantity used to describe the energy available to do work in a system
The concept of free energy is important in many areas of chemistry, including chemical thermodynamics, biochemistry, and materials science. It is used to understand and predict the behavior of chemical reactions, phase transitions, and other thermodynamic processes.
ΔG = ΔG° + RTlnQ
ΔG = 3.2 * 10^3 + (8.314 * 295 * ln(3.3)
= 3.2 * 10^3 + 2.928 * 10^3
= 6.128 * 10^3 J/mol
or 6.128 kJ/mol
Learn more about free energy:https://brainly.com/question/15319033
#SPJ1
Given the following skeleton equation, what are the coefficients for the chemicals in the reaction? __H20 + __ P4O10 = __ H3PO4
Answers
Coefficients: 6, 1, 4
How can an igneous rock turn into a sedimentary rock?
Answers
Answer:
On the surface, weathering and erosion break down the igneous rock into pebbles, sand, and mud, creating sediment, which accumulates in basins on the Earth's surface. As successive layers of sediment settle on top of one another, the sediment near the bottom is compressed, hardens, and forms sedimentary rock.6. A sample of a gas at 77°C and 1.33 atm occupies a volume of 50.3 L. How many moles of the gas are present? (Hint: Since moles have been asked, which equation has the moles listed in the equation. Use that to solve this problem).
Answers
The number of moles of the gas at 77°C and 1.33 atm occupies a volume of 50.3 L is 2.35 moles. It can found with the help of Ideal gas equation.
What is Ideal Gas equation ?The ideal gas equation is formulated as : PV = nRT.
In this equation, P refers to the pressure of the ideal gas, V is the volume of the ideal gas, n is the total amount of ideal gas that is measured in terms of moles, R is the universal gas constant, and T is the temperature.
Given ;
Pressure = 1.33 atmVolume = 50.3 ltrTemperature = 77 (+273 k) = 350KWe know ;
Gas constant (R) = 0.081 L atm/mol KFormula used ;
n = PV / RT
n = 1.33 x 50.3 / 0.081 x 350k
= 2.35 moles.
Hence, The number of moles of the gas at 77°C and 1.33 atm occupies a volume of 50.3 L is 2.35 moles
Learn more about Ideal Gas here ;
https://brainly.com/question/27922399
#SPJ1
Please Help!!50 points and I’ll mark as brainliest!
Tasks are in the picture.

Answers
1) The pH is 2.5
2) The pH is 11.5
3) The initial concentration is\(2.1 * 10^-14\)M
What is the pH?pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the concentration of hydrogen ions in the solution.
1) The pH of the solution can be gotten from;
K = \(x^2\)/0.65 - x
\(1.754 * 10^-5\)(0.65 - x) = \(x^2\)
\(1.14 * 10^-5 - 1.754 * 10^-5x = x^2\\x^2 + 1.754 * 10^-5x - 1.14 * 10^-5 = 0\)
x = 0.003 M
pH = -log(0.003)
= 2.5
2) Kb = \(x^2\)/0.35 - x
\(1.8 * 10^-5 (0.35 - x) = x^2\\6.3 * 10^-6 - 1.8 * 10^-5x = x^2\\x^2 + 1.8 * 10^-5x - 6.3 * 10^-6 = 0\)
x = 0.003 M
pOH = -log (0.003)
= 2.5
pH = 14 - 2.5 = 11.5
3) Hydrogen ion concentration = Antilog (-11.5)
= 3.2 * 10^-12 M
\(4.9 * 10^-10 = ( 3.2 * 10^-12)^2/x \\4.9 * 10^-10x = ( 3.2 * 10^-12)^2\\x = ( 3.2 * 10^-12)^2/4.9 * 10^-10\\x = 2.1 * 10^-14 M\)
Learn more about pH:https://brainly.com/question/29766400
#SPJ1
15 points help
A student is labeling a model of the cell cycle. Which substance(s) should the student label as
regulating the cell cycle?
A. Chromosomes
B. Cyclins
C. Nutrients
D. DNA
E. RNA
Answers
Answer:
A. chromosomes
Explanation:
chromosomes because the baby of the womb take some setting features from their parent
conclusion for polarity of liquid
Answers
Answer:
The polarity of a liquid refers to the separation of electric charge within the molecules of the liquid, resulting in a positive and negative end. Based on this, we can draw the following conclusion:
In conclusion, the polarity of a liquid is an important property that affects its behavior and interactions with other substances. Polar liquids have molecules with an uneven distribution of charge, resulting in positive and negative ends. This polarity influences various aspects, such as solubility, surface tension, and the ability to dissolve other polar substances. Additionally, polar liquids tend to exhibit stronger intermolecular forces, leading to higher boiling and melting points compared to nonpolar liquids. Understanding the polarity of a liquid is crucial for various fields, including chemistry, biology, and material science, as it helps explain and predict the behavior and properties of different substances in a wide range of applications.
PLEASE MARK AS BRAINLIESTAnswer:
The polarity of a liquid refers to the separation of electric charges within the molecule, resulting in a molecule with a positive end and a negative end. The presence or absence of polarity in a liquid has significant implications for its behavior and interactions with other substances.
In conclusion, the polarity of a liquid plays a crucial role in determining its physical and chemical properties. Polar liquids, such as water, have an unequal distribution of charge within their molecules, leading to hydrogen bonding and strong intermolecular forces. These interactions give rise to properties like high boiling points, surface tension, and solubility, making polar liquids excellent solvents and essential for many biological processes.
On the other hand, nonpolar liquids, such as hydrocarbons, have a symmetrical distribution of charge and lack strong intermolecular forces like hydrogen bonding. As a result, they have lower boiling points, weaker interactions, and are typically less soluble in polar solvents. Nonpolar liquids are commonly used as solvents for nonpolar compounds and have different applications in various industries.
Understanding the polarity of a liquid is crucial in fields such as chemistry, biology, and materials science. It helps predict how substances will interact and dissolve in a given solvent, as well as how they will behave in chemical reactions. Additionally, polarity affects the physical properties of liquids, including their viscosity, conductivity, and surface behavior.
In summary, the polarity of a liquid is a fundamental characteristic that influences its behavior, solubility, and reactivity. Whether a liquid is polar or nonpolar has far-reaching consequences in various scientific disciplines and practical applications
Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size.
atoms or lons:
•Be, Be^+, Li
•Br, I, Cl
•Cl, Na, S
atoms or lons in order
of decreasing size

Answers
Answer: Na, S, Cl
Explanation:
Atomic size decreases as one moves from left to right on the periodic table with elements in the same period. This is as a result of the electrons increasing in the outer circle and thus being drawn to the protons in the nucleus which will lead to the outer shell area decreasing.
Sodium (Na) comes before Sulfur (S) which comes before Chlorine (S) so this is the decreasing order as they are all in the same period.
Name the following alkyne: CH3CH₂C = CCH₂CH₂CH3
B. 3-heptyne
D. 3-heptene
A. 4-heptyne
C. 3-heptane
Answers
The given alkyne is Option A 3-heptyne
What is an Alkyne ?The hydrocarbon having at least one C-C triple bond is called an Alkyne.
It has the general formula of \(\rm C_n H_{2n+2}\) .
In the question it is being mentioned that it is an alkyne so there will be a triple bond and not a double bond.
It has been asked in the question that
CH3CH₂C ≡ CCH₂CH₂CH3 is which alkyne from the given option.
The counting of the Carbon chain is done from the left side and the Triple bond is at the 3rd Carbon , so 3-heptyne .
To know more about Alkyne
https://brainly.com/question/23508203
#SPJ1
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
Task:
For each "station", click on the link. You should describe the initial appearances and observations of the
reaction during and after. Using your observations, determine if the change is a physical or chemical change.
Station #1: Lead Nitrate and Potassium lodide solutions. Shower of yellow
QUESTION/OBSERVATION
INITIAL APPEARANCE (what does the
substance look like in the beginning)
Answers
The expected observations for the chemical reaction involving lead nitrate and potassium iodide are as follows as per theory.
INITIAL APPEARANCE:Before the reaction, you'd have two separate solutions:
Lead Nitrate solution: This is typically a clear, colorless solution.
Potassium Iodide solution: This is also usually a clear, colorless solution.
REACTION OBSERVATIONS:
As soon as you combine these two solutions, a chemical response takes place, resulting in the almost instantaneous development of a yellow precipitate. Lead iodide is a substance that cannot be dissolved in water.
FINAL APPEARANCE:
The final mixture would have a yellow precipitate (lead iodide) suspended in the solution.
The reaction leads to the formation of lead iodide, a substance with distinctive properties, suggesting a chemical change. The presence of this novel compound is indicated by the yellow hue of the precipitate.
Read more about chemical change here:
https://brainly.com/question/1222323
#SPJ1
Choose the correct option.
1. A chain of small chemical units combined to form a large single unit is called ______
a) Polymer
b) Poly
c) Polythene
d) None of the above
2. Polythene and PVC are examples of
a) Bio degradable substance
b) Thermosetting plastics
c) Thermoplastics
d) Rayon
3. Plastics which when moulded once, cannot be softened by heating. Such plastics are called __ a) Polythene
b) Thermoplastics
c) Polyster
d) Thermosetting plastics
4. Polycot is made by mixing two types of fibres namely
a) Silk + Cotton
b) Polythene + cotton
c) Silk + Polyester
d) Polyester + Cotton
5. The 4 R Principle is
a) Reduce, Reuse, Recycle, Recover
b) Remember, reduce, Recycle, Rejoice
c) Repeat, Rejoice, recycle, reduce
d) None of the above
6. _____________ is an example of natural polymer
a) Rayon
b) Cellulose
c) Nylon
d) All of the above
7. Which of the following is Non-biodegradable ?
a) Woolen clothes
b) Plastic bag,
c) Cotton cloth
d) Wood
8. Bakelite and Melamine are examples of
a) Thermosetting plastics
b) Silk
c) Nylon
d) Rayon
9. Fire proof plastic uniform worn by fire fighters has a coating of _____ to make it fire resistant. a) Nylon
b) Rayon
c) Melamine plastic
d) silk
The coating on modern non- stick cookware and electric iron is of
a) Terrycot
b) Rayon
c) Polyester
d) Teflon
Answers
2 Thermoplastic
3 Thermosetting plastics
4 polyester + cotton
5 Reduce, reuse, recycle, recover
6. Cellulose
7 wood
8 thermosetting plastic
9silk
10 Teflon
Need help with this please it’s due today
Write agree or disagree next to each statement. Explain why you agree or why you disagree
1. Metal atoms don’t have full vallance electron shells, so they try to gain electrons to fill their outer shell.
2. When atoms join together,the bond is either covalent or ionic
3. Oxygen atoms bond together,but this is not because of an atomic force.
4. Covalent bonds happen when an electron moves back and forth between two atoms
5. Ionic bonds occur when an electron is transferred from one atom to another
6. When hydrogen joins with oxygen,a hydrogen bond has been formed.
Answers
Answer:
The statements 1, 2, 5 are correct, while the statements 3, 4, 6 are incorrect. It's important to have a clear understanding of the different types of chemical bonds and how they are formed in order to understand the properties and behavior of molecules and compounds.
Explanation:
1. Agree: Metal atoms have partially filled valence shells, which makes them more likely to lose electrons than to gain them. This loss of electrons creates a positive ion with a full valence shell, which is more stable than the original atom.
2. Agree: Chemical bonds are either covalent or ionic. Covalent bonds involve the sharing of electrons between atoms, while ionic bonds involve the transfer of electrons from one atom to another.
3. Disagree: Oxygen atoms bond together due to a strong atomic force called a covalent bond. This occurs when the two oxygen atoms share electrons to form a stable molecule of O2.
4. Disagree: Covalent bonds occur when electrons are shared between atoms, but they do not "move back and forth" between the atoms. Rather, the electrons are shared between the atoms to create a stable molecule.
5. Agree: Ionic bonds occur when one atom transfers an electron to another atom. The transfer of the electron creates two ions with opposite charges, which are then attracted to each other.
6. Disagree: A hydrogen bond is a weak electrostatic attraction between a hydrogen atom and an electronegative atom such as oxygen or nitrogen. When hydrogen joins with oxygen, a covalent bond is formed, not a hydrogen bond.
Metal atoms try to gain electrons; Covalent or ionic bonds form when atoms join; Oxygen atoms bond via atomic force.
Explanation:1. Agree - Metal atoms do not have full valence electron shells, so they tend to gain electrons to achieve stability. This process is known as electron transfer or ionic bonding.
2. Agree - when atoms join together, the resulting bond is either covalent or ionic. Covalent bonds involve the sharing of electrons, while ionic bonds involve the transfer of electrons from one atom to another.
3. Disagree - Oxygen atoms bond together through a force known as the atomic force. This force arises due to the different electronegativities of the oxygen atoms, leading to the formation of a covalent bond.
4. Disagree - Covalent bonds occur when electrons are shared between atoms, rather than moving back and forth between them.
5. Agree - Ionic bonds occur when one atom transfers an electron to another atom.
6. Disagree - When hydrogen joins with oxygen, a covalent bond is formed, not a hydrogen bond. Hydrogen bonds occur when a hydrogen atom is attracted to an electronegative atom, such as oxygen, in a different molecule.
Learn more about Chemical bonding here:https://brainly.com/question/33579397
#SPJ2
Compare the polymerisation reaction used to produce poly(ethene) with the
polymerisation reaction used to produce a polyester.
Answers
Answer:
See explanation
Explanation:
Polymerization is the process whereby two or more monomers link together to form a compound of high molecular mass called a polymer.
There are two kinds of polymers;
-Addition polymers
-Condensation polymers
Addition polymers are formed by the joining of two or more monomers to form a polymer without the elimination of a small molecule.
Condensation polymers are formed by the joining of two or more molecules to form polymers with the elimination of a small molecule.
The main difference between polyethene and polyester is that polyethene is an addition polymer while polyester is a condensation polymer.
In polyethene, ethene molecules are joined together having the repeating unit as [-CH2-CH2-]n.
In polyester, the polymer arises from the reaction of carboxylic acid and an alcohol and loss of water molecules.
The comparison of the polymerisation reaction should be described below:
Polymerisation:It is the process in which two or more monomers are connected together to create a high molecular mass compound that we called as the polymer.
It comprises two polymers. Addition polymers should be connected by the linking of two or more monomers to create a polymer without removing a small molecule.
While on the other hand, Condensation polymers should be developed by the connecting of two or more molecules to creating polymers with the removing of a small molecule.
The main difference that lies between polyethylene and polyester is that polyethylene should be an additional polymer while on the other hand polyester is a condensation polymer.
Learn more about the reaction here: https://brainly.com/question/24752692
Which one is a decomposition reaction?
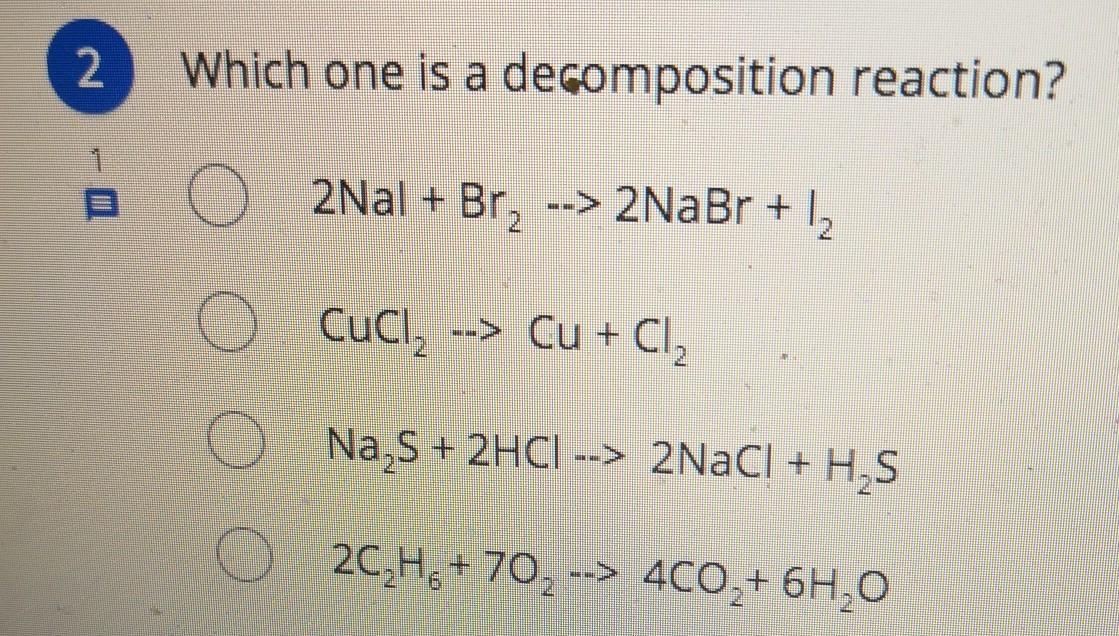
Answers
Answer:
b no
Explanation:
because it is decomposing into two elements
A sample of silver (Ag) contains 3.011x1023 atoms. How much does this
sample weigh (in g)?
is out of
Answer:
Answers
Answer:
53.93 g Ag
Explanation:
3.011x1023 atoms Ag x 1 mole Ag x 107,8683 grams =
6.022x\(10^{23}\) atoms 1 mole Ag
use the periodic table fo find Ag atomic mass and use Avogadro´s number
A mass of 6.005 g of carbon (atomic mass 12.010 amu) contains...? ty in advance
Answers
The mass of 6.005 g of carbon contains approximately 3.011 x 10²³ carbon atoms.
How much mass do six moles of carbon atoms have?We are aware that a mole is a grouping of 6.022 10²³ atoms. 6.0221023 carbon atoms make up a mole of carbon. As a result, we can estimate that 6.0221023 carbon atoms have a mass of 12 grammes.
There are: atoms of carbon in the sample.
The amount of carbon atoms in the sample may be determined using Avogadro's number (6.022 x 10²³ atoms per mole) and the molar mass of carbon (12.010 g/mol)
Number of moles of carbon = mass of carbon/molar mass of carbon
= 6.005 g / 12.010 g/mol
= 0.500 mol
Number of carbon atoms=number of moles of carbon x Avogadro's number
= 0.500 mol x 6.022 x 10²³ atoms/mol
= 3.011 x 10²³ atoms
To know more about carbon visit:-
https://brainly.com/question/22530423
#SPJ1
Calculate the work done when a force of 4 N pulls a box along the floor for a distance of 0.3 m.
Answers
Answer:
1.2 Joulesolution,
Force=4 N
Distance=0.3 m
Now,
\(work = f \times d \\ \: \: \: \: \: \: \: \: \: \: \: = 4 \times 0.3 \\ \: \: \: \: \: \: \: \: \: \: \: = 1.2 \: joule\)
hope this helps...
Good luck on your assignment....
Draw a diagram showing the arrangement of the valency electrons around
atoms in a Carbon tetrachloride molecule, CCl4
Use ‘o’ to represent an electron of carbon and ‘x’ to represent an electron of
Chlorine
BRAINLIEST
Answers
But there is no any option for sending pic.
7. Intermolecular forces hold the oxygen and hydrogen atoms together in a water molecule.
a. TRUE
b. FALSE
Answers
The reaction 2A → A2 was experimentally determined to be second order with a rate constant, k, equal to 0.0265 M–1min–1. If the initial concentration of A was 5.00 M, what was the concentration of A (in M) after 180.0 min?
Answers
The concentration is 0.00372 M.
What is a second order reaction?A second-order reaction is a chemical reaction in which the rate of the reaction is proportional to the square of the concentration of one or two reactants. In other words, the reaction rate is directly proportional to the product of the concentrations of the reactants raised to the power of two.
A second-order reaction can be expressed mathematically as:
rate = k[A]^2 or rate = k[A][B]
We have that;
1/[A]t = kt + 1/[A]o
1/[A]t =(0.0265 * 180 * 60) + 1/5
[A]t= 0.00372 M
Learn more about second order:https://brainly.com/question/12446045
#SPJ1