Answers
Related Questions
Which of the following contains the atom with the highest oxidation number?
a. NaClO4
b. FeCl3
c. H2O2
d. SnH4
e. CrO3
Answers
The compound that contains the atom with the highest oxidation number is CrO₃ (option E).
What is oxidation number?Oxidation number is the hypothetical charge of an atom within a molecule or compound.
The oxidation number of an atom or ion determines the subscript given to the other elements in the molecule.
According to this question;
in the molecule: CrO₃, chromium has an oxidation number of +6 while oxygen has an oxidation number of -2. in the molecule: FeCl₃, iron has an oxidation number of +3 while chlorine has an oxidation number of -1.Therefore, it can be said that the molecule that posseses the atom with the highest oxidation number is CrO₃.
Learn more about oxidation number at: https://brainly.com/question/15167411
#SPJ1
HI(aq)+H2O(l)→ H3O+(aq)+I−(aq)
Match the words to the appropriate blanks in the below sentences.
a. The Bronsted-Lowry ace is :_________
b. The Bronsted-Lowry base is :_________
c. The conjugate acid is :_________
d. The conjugate base is:________
1. H2O(l)
2. HI(aq)
3. I^-
4. H2O^+
Answers
Answer:
Given chemical reaction is:
HI(aq)+H2O(l)→ H3O+(aq)+I−(aq)
a. The Bronsted-Lowry ace is :_________
b. The Bronsted-Lowry base is :_________
c. The conjugate acid is :_________
d. The conjugate base is:________
Explanation:
According to Bronsted-Lowry acid-base theory,
an acid is a substance, that is a proton donor.
A base is a proton acceptor.
The conjugate acid is formed from the base after gaining a proton.
The conjugate base is formed from the acid after losing a proton.
For the given reaction,
a. The Bronsted-Lowry acid is :__HI(aq)_______
b. The Bronsted-Lowry base is :_H2O(l)________
c. The conjugate acid is :___H3O+(aq)______
d. The conjugate base is:___I-(aq)_____.
Answer:
Explanation:
Bronsted -Lawry acid are hydrogen ion donators . Here HI is Bronsted -Lawry acid.
HI ⇄ H⁺ + I⁻
Bronsted -Lawry base are those which can accept hydrogen ion .
I⁻ + H⁺ ⇄ HI .
Conjugate acid -base pair are shown below .
H₃O⁺ = H⁺ + H₂O .
conjugate acid conjugate base .
( strong acid ) ( weak base )
500.0 mL of a 0.205 M solution of LiBr is diluted to 700.0 mL. What is the new concentration of the solution?
Answers
Answer:
0.146 M
Explanation:
Use v1s1 = v2s2
here, v1 = 500 mL, v2 = 700 mL, s1 = 0.205 M & s2 = new concentration
how to solve x² in differential
Answers
Answer:
x² = mutiphy by them self
Explanation:
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
1. What mass (in g) of nitrogen are needed to react completely with 5.8 g of hydrogen?
N2 + 3H2 + 2NH3
5.8 g N2
81 g N2
76 g N2
27 g N2
Answers
Answer:
27g N2
Explanation:
FILL IN THE BLANK. Whereas sodium is found mainly in the extracellular fluid, most ______ is found in the intracellular fluid. A) iron. B) chloride. C) bicarbonate
Answers
The sodium is found mainly in the extracellular fluid, most chloride is found in the intracellular fluid.
What is intracellular fluid?Intracellular fluid (ICF) is the liquid contained within cells in the body. It represents approximately two-thirds of the body's total fluid volume and is responsible for maintaining the proper functioning of cells. The ICF provides the cells with nutrients and oxygen, helps remove waste products, and helps regulate the cells' internal environment, including temperature and pH. The composition of the ICF is different from the extracellular fluid (ECF) that surrounds the cells and includes a variety of ions, such as potassium, magnesium, and phosphate, as well as proteins and other molecules. The ICF and ECF work together to maintain the body's overall fluid balance and support its various physiological functions.
To know more about extracellular fluid, visit:
https://brainly.com/question/14831457
#SPJ1
When looking at a sample of vegetable oil, what biomolecule would there be a lot of?
A. Nucleic Acids
B. Proteins
C. Carbohydrates
D. Lipids
Answers
Answer: D. Lipids. Vegetable oils are composed mainly of lipids, which are fatty acids and glycerol.
Explanation:
Imagine running one hour straight west at 5 km/h and then changing direction quickly and running
one hour straight north at 5 km/h. What was your total displacement? Round to the nearest whole
number.
O 10 km
O 5 km
O 50 km NW
O 7 km NW
Answers
Answer:
If you ran one hour straight west at 5 km/h and then changed direction quickly and ran one hour straight north at 5 km/h, your total displacement would be 7 km NW.
Explanation:
This is because your total change in position would be 5 km to the west and 5 km to the north, resulting in a displacement of 7 km NW.
After running one hour straight west at 5 km/h and then changing direction quickly and running one hour straight north at 5 km/h, the total displacement would be 7 km NW, rounded to the nearest whole number.
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
1. what is an atom
2. what is a chemical process
3. WHat is a chemical reaction
Answers
Answer:
1.An atom is the defining structure of an element, which cannot be broken by any chemical means. A typical atom consists of a nucleus of positively-charged protons and electrically neutral neutrons with negatively-charged electrons orbiting this nucleus.
2.a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds.
3. a process that involves rearrangement of the molecular or ionic structure of a substance, as opposed to a change in physical form or a nuclear reaction.
Which data set is precise based on a correct value of 42?
Set 1: 41, 23, 42, 19
Set 2: 41, 42, 41, 43
Set 3: 42, 15, 42, 98
Set 4: 89, 13, 17, 25
Answers
Answer:
2
Explanation:
A crop is sprayed with a pesticide to prevent infestation and damage from insects. However, the next season the same pesticide fails to prevent the insects from damaging the crop. Why
Answers
Answer:
Farmers spray to mitigate crop damage caused by pests. A pest is any biological organism, including weeds, pathogens, and arthropods, that interferes with the production of crops affecting quality and/or yield. ... Pesticides work in many different ways by affecting their target, whether it be a weed, pest, or disease.
Explanation:
this is my answer❤︎
Select the correct answer.
Which of the following does the Sun orbit?
A.
The center of the Milky Way
B.
The Earth
C.
The Moon
D.
The stars
Answers
Explanation: It's located about two-thirds of the way out from the center of the Milky Way which is about 28,000 light–years away.
What is a combination of two or more substances that are all solids
Answers
Answer:
Solids cant react to each other because they are the same room temperature
Answer:
A is the correct answer.
Explanation:
A mixture of solids is the correct answer.
Sue dissolved a certain amount of salt in 400 grams of water to obtain 405 grams of salt solution. What was the mass of the salt used to make the solution? 5 grams 10 grams 400 grams 405 grams
Answers
Explanation:
Sue added salt to water in order to make a salt solution
the original amount of water is 400 grams
In order for Sue to obtain 405 grams of salt solution using 400 grams of water, 5 grams of salt must be added.
Answer: (D) 5 grams
Explanation: the reason I would chose this is because if you do the simple math of 405-400 you get 5 so if he has 400 in the beginning he would have to add something to make it 405 grams and that something would have to be 5 grams of salt. hoped this helped no need for brainiest just happy to help.:)
and please tell me if I am wrong.
What system works most directly with the nervous and skeletal system to move the body?
A.
immune
B.
muscular
C.
excretory
D.
cardiovascular
Answers
Answer:itsB
Explanation:
The muscular system works most directly with the nervous and skeletal systems to move the body. Thus, option (B) is correct.
What is the muscular system of the human body?The muscular system is made up of various kinds of muscles that each play an important role in the function of the human body. Only skeletal muscles can be consciously controlled. They are attached to bones in our body and cause movement of those bones.
Any action which is consciously undertaken involves the use of skeletal muscles. Examples include chewing, running, and writing.
Smooth muscle lies inside blood vessels and organs such as the stomach. These are the weakest type of muscle but have a crucial role in moving food and maintaining blood circulation.
The cardiac muscle regulates its own contractions that generate our heartbeat. Signals from the human nervous system control the rate of contraction. These muscles act involuntarily and are strong.
Learn more about the muscular system, here:
https://brainly.com/question/3162365
#SPJ2
What would make oppositely charged objects attract each other more?increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged objectdecreasing the positive charge of the positively charged object and decreasing the negative charge of the negatively charged objectincreasing the distance between the positively charged object and the negatively charged objectmaintaining the distance between the positively charged object and the negatively charged object
Answers
Answer:
increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged object would make the oppositely charged objects attract each other more.
whats the mass of 4.35x 10^-2 mol of NAOH
Answers
The mass of 4.35 × 10^-2 mol of NaOH is 1.74 grams.
Given
Number of moles = 4.35 x 10^-2
First, we calculate the molar mass of NaOH,
Molar mass of NaOH = (1 × atomic mass of Na) + (1 × atomic mass of O) + (1 × atomic mass of H)
= (1 × 22.99 g/mol) + (1 × 16.00 g/mol) + (1 × 1.01 g/mol) = 40.00 g/mol
Molar mass of NaOH = 40.00 g/mol
Mass of NaOH = Number of moles × Molar mass
= 4.35 × 10^-2 mol × 40.00 g/mol
Mass of NaOH = 1.74 g
Numericals on Molar Mass :
https://brainly.com/question/19461013
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
what is the litosphere made of
Answers
Answer:
brittle crust and top is made of upper mantel
Example A vegetable are chopped Example B food is broken up into simpler form during digestion which statement is correct
Answers
Both Example A and Example B are correct, but they refer to different processes. Example A refers to the physical action of chopping vegetables into smaller pieces, which can make them easier to cook and eat.
This process does not change the fundamental nature of the vegetable, but simply alters its physical properties.
Example B, on the other hand, refers to the process of digestion in the human body. During digestion, food is broken down into simpler forms, such as sugars, amino acids, and fatty acids, which can be absorbed and used by the body for energy and other functions. This process involves both physical and chemical changes, as enzymes in the digestive system break down complex molecules into simpler ones.
In summary, Example A describes a physical process that alters the size and shape of a vegetable, while Example B describes a physiological process that breaks down complex food molecules into simpler forms for use by the body. Both processes are important for preparing and consuming nutritious food.
for more such question vegetables
https://brainly.com/question/18134846
#SPJ11
burning 12g of urea raise temp of water by 30C what is the enthalpy of combustion for 1kg urea
Answers
The enthalpy of combustion for 1kg of urea is -1223525.84 J/mol.
Urea is a compound that is used in fertilizers and in some plastics.The enthalpy of combustion for urea is the amount of energy that is released when urea is burned. In order to calculate the enthalpy of combustion for 1kg of urea, we need to use the information that is provided to us in the question. Let us start by writing down the balanced equation for the combustion of urea: CO(NH2)2 + 3/2 O2 → CO2 + 2H2O + N2
The balanced equation shows that 1 mole of urea reacts with 1.5 moles of oxygen gas to produce 1 mole of carbon dioxide, 2 moles of water, and 1 mole of nitrogen gas. The enthalpy change for this reaction is equal to the amount of energy that is released when 1 mole of urea is burned.
The heat of combustion (ΔHc) of urea is -632.6 kJ/mol. This means that 632.6 kJ of energy is released when 1 mole of urea is burned. We know that 12g of urea raised the temperature of water by 30°C. We can use this information to calculate the amount of energy that was released when 12g of urea was burned.
The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 J of energy to raise the temperature of 1 gram of water by 1°C. Therefore, it takes 4.18 x 1000 = 4180 J of energy to raise the temperature of 1 kg of water by 1°C.
We know that 12g of urea raised the temperature of water by 30°C. Therefore, the amount of energy that was released when 12g of urea was burned is:
Energy = mass x specific heat capacity x temperature change
Energy = 0.012 kg x 4180 J/kg°C x 30°C
Energy = 1497.6 J
We can now use this information to calculate the enthalpy of combustion for 1kg of urea:
Enthalpy of combustion = energy released / moles of urea burned
Enthalpy of combustion = 1497.6 J / (0.012 kg / 60.06 g/mol)
Enthalpy of combustion = - 1223525.84 J/mol
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
Please Help!! Balancing Redox Reactions Worksheet questions 4-7 (see attached)
Answers
The balanced redox reaction in the chemical reaction is given below:
40H2S + 48H+ + 16MnO4¯ ---> 5S8 + 16Mn2+ + 64H2O
Balancing the redox reaction:
Solution:
1) Half-reactions:
H2S ---> S8
MnO4¯ ---> Mn2+
2) Balance:
8H2S ---> S8 + 16H+ + 16e¯
5e¯ + 8H+ + MnO4¯ ---> Mn2+ + 4H2O
3) Make the number of electrons equal (note that there are no common factors between 5 and 16 except 1):
40H2S ---> 5S8 + 80H+ + 80e¯ <--- factor of 5
80e¯ + 128H+ + 16MnO4¯ ---> 16Mn2+ + 64H2O <---
factor of 16
4) Thus, the final answer is given below;
40H2S + 48H+ + 16MnO4¯ ---> 5S8 + 16Mn2+ + 64H2O
What is oxidation-reduction reaction?Oxidation-reduction can simply be defined as a special type of chemical reaction in which the oxidation states of the substrate change.
So therefore, the balanced redox reaction in the chemical reaction is given below:
40H2S + 48H+ + 16MnO4¯ ---> 5S8 + 16Mn2+ + 64H2O
Complete question:
Balance the following redox reaction:
MnO4¯ + H2S ---> Mn2+ + S8
Learn more about oxidation-reduction:
https://brainly.com/question/21851295
#SPJ1
Calculate the mass of NaCO3 used in experiment. SHOW WORK — 15 points!!
Mass of empty evaporating dish: 46.233g
Mass of evaporating dish + sodium bicarbonate: 48.230g
Mass of evaporating dish + product after 1st drying: 47.504 g
Mass of evaporating dish: 46.233g
Mass of evaporating dish + product after 2nd drying: 47.485
Answers
The mass of sodium bicarbonate (NaHCO₃) used in the experiment is 1.997 g
Calculating massFrom the question we are to calculate the mass of NaHCO₃ (sodium bicarbonate) used in the experiment
From the given information
Mass of empty evaporating dish = 46.233g
Mass of evaporating dish + Sodium bicarbonate = 48.230g
∴ Mass of sodium bicarbonate (NaHCO₃) = [Mass of evaporating dish + Sodium bicarbonate] - [Mass of empty evaporating dish]
Mass of sodium bicarbonate (NaHCO₃) = 48.230g - 46.233g
Mass of sodium bicarbonate (NaHCO₃) = 1.997 g
Hence, the mass of sodium bicarbonate (NaHCO₃) used in the experiment is 1.997 g
Learn more on Calculating mass here: https://brainly.com/question/15268826
how to calculate theoretical yield
Answers
Answer:
I'm not really sure how but here's the formula?

To calculate theoretical yield first check chemical equations are balanced. Calculate the mole ratios of the reactants and products, Find the theoretical yield of the reaction.
Percent Yield = Mass of Actual Yield / Mass of Theoretical Yield x 100 percent.
What is theoretical yield ?The theoretical yield is the quantity of product that stoichiometry predicts will be produced, whereas the actual yield is the amount that is actually produced.
The yield of a reaction is used to represent how much of a product is produced from that reaction.
Thus, Divide the ratio by the limiting reactant's molecular weight. The answer is the theoretical yield of the desired product in moles.
To learn more about the theoretical yield, follow the link;
https://brainly.com/question/14966377
#SPJ1
Give me an example of an average. For instance, "the average height of a person is about 5
foot 6 inches".
Answers
A hospital saline solution is analyzed to confirm its concentration. A 50.0 mL sample with a mass of 50.320 g is evaporated to dryness. If the solid sodium chloride residue has a
mass of 0.669 g. what is the mass percent and molar concentration of the saline solution?
Answers
The mass percent of the sodium chloride in the saline solution is approximately 1.33%. The molar concentration of the saline solution is approximately 0.229 M.
To determine the mass percent and molar concentration of the saline solution, we need to analyze the mass of the sodium chloride residue and the initial mass of the sample.
Mass percent:
The mass percent is calculated by dividing the mass of the sodium chloride residue by the initial mass of the sample and then multiplying by 100%.
Mass percent = (Mass of NaCl / Initial mass of sample) × 100%
Mass of NaCl = 0.669 g
Initial mass of sample = 50.320 g
Mass percent = (0.669 g / 50.320 g) × 100% ≈ 1.33%
The mass percent of the sodium chloride in the saline solution is approximately 1.33%.
Molar concentration:
To calculate the molar concentration of the saline solution, we need to determine the number of moles of sodium chloride and the volume of the solution.
Moles of NaCl = Mass of NaCl / Molar mass of NaCl
The molar mass of NaCl is 58.44 g/mol.
Moles of NaCl = 0.669 g / 58.44 g/mol ≈ 0.01144 mol
Since the volume of the sample is given as 50.0 mL, we need to convert it to liters.
Volume of solution = 50.0 mL = 50.0 mL × (1 L / 1000 mL) = 0.0500 L
Now we can calculate the molar concentration (Molarity) using the formula:
Molarity (M) = Moles of solute / Volume of solution (in liters)
Molarity = 0.01144 mol / 0.0500 L ≈ 0.229 M
The molar concentration of the saline solution is approximately 0.229 M.
for more such questions on concentration
https://brainly.com/question/17206790
#SPJ11
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
All the parts are connected to each other however have their own answers
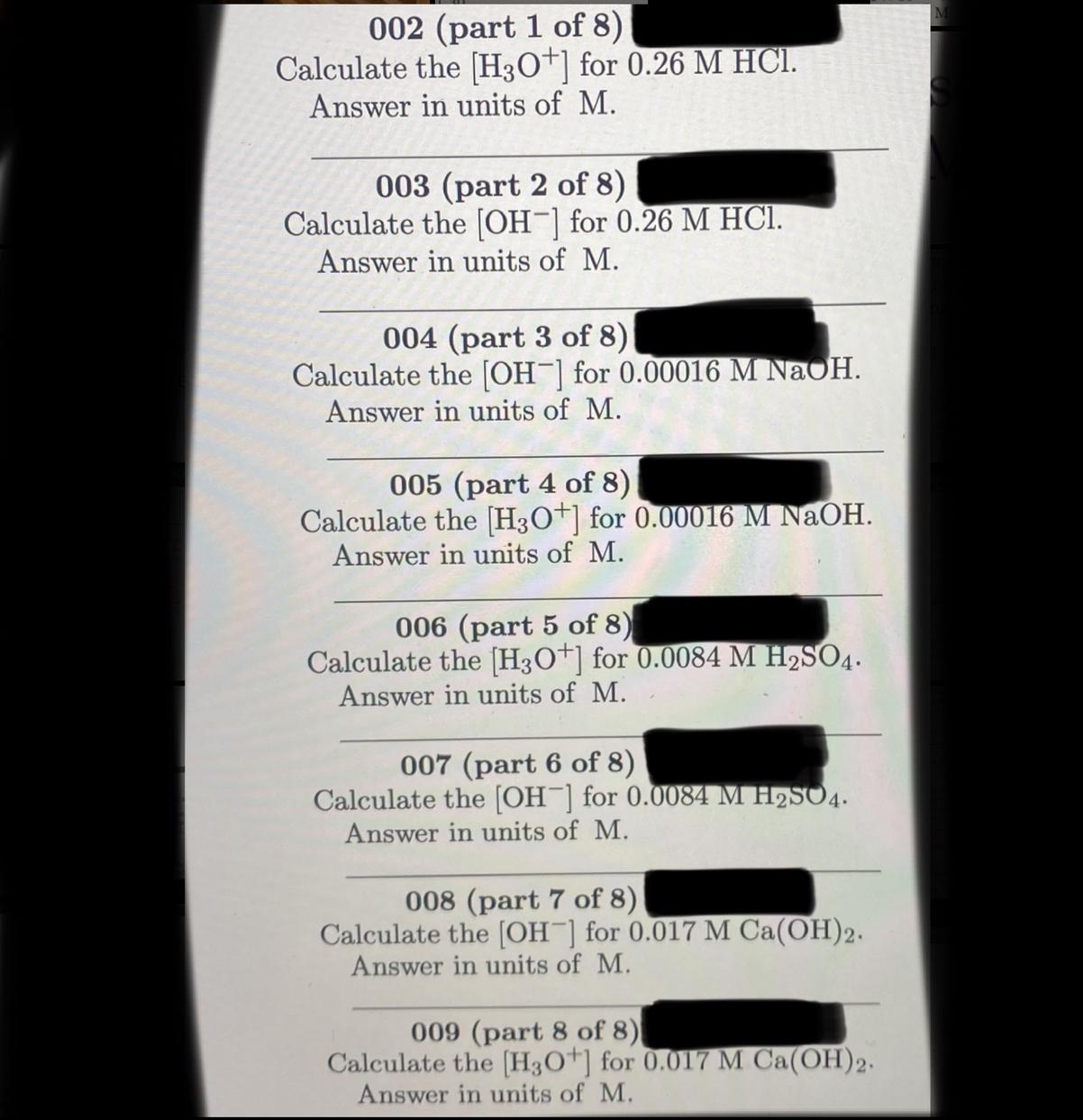
Answers
Calculate H3O+
1) Write the chemical equation
\(\text{HCl}_{(aq)}\rightarrow H^++Cl^-\)2) Calculate H3O+
\(\lbrack H_3O^+\rbrack=0.26M\cdot\frac{1MH_3O^+}{1\text{M HCl}}=0.26MH_3O^+\)The H3O+ solution is 0.26M.