Answers
Response: N2 + 2O2 = 2NO2.
An entire or partial burning of items occurs during a combustion event when oxygen is present.
The appropriate combustion reaction in this instance is N2(g) + 2O2(g) 2NO2 (g)
The reaction demonstrates how nitrogen gas burns in the air to create nitrogen (IV) oxide.
Precipitation reactions, decomposition reactions, synthesis processes, single and double replacement reactions, among others, are different types of chemical reactions.
Study up on Synthesis reactions.
Visit brainly.com/question/16987748
#SPJ1
Related Questions
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
Which of the following can be characterized as a physical change?
A. a copper pipe rusts
B. milk goes sour
C. firewood burns
D. sweat evaporates from your skin
Answers
Answer:
D. Sweat evaporates from your skin
Explanation:
A physical change is a reversible process, no new substance is formed. A physical change does not affect the chemical composition of a substance.
Sweat evaporation from the skin is a physical change, because the change that occur is merely a change of state, Liquid to Gaseous state, no new substance is formed.
What should you do if your hypothesis proves false during an experiment?
A change the data to fit the hypothesis
B form a new hypothesis and plan a new experiment
C repeat the experiment until the results support the hypothesis
D change the procedure to get the
results you want
Answers
D because you can easily make mistakes but keep goin to get it right.
A gas expands from a volume 2.0 L at 36oC to a volume of 2.5 L, what is the final temperature, if the pressure is constant?
Answers
Answer:
386.45 K
Explanation:
We can solve this problem by using Charles' law, which states that at constant pressure:
V₁T₂=V₂T₁Where subscript 1 stands for initial conditions of volume and temperature, while 2 stands for the final ones. Meaning that in this case:
V₁ = 2.0 LT₂ = ?V₂ = 2.5 LT₁ = 36 °C ⇒ 36 +273.16 = 309.16 KWe input the data:
2.0 L * T₂ = 2.5 L * 309.16 KAnd solve for T₂:
T₂ = 386.45 KWrite the chemical symbols for three different atoms or atomic anions with 23 electrons.
Answers
Krypton, Chromium, and Oxygen with the following symbols Kr-13, Cr-2, and O-15 respectively have 23 electrons.
The atomic number of an atom determines the number of electrons it has. When the number of protons is equivalent to the number of electrons, the atom is electrically neutral. An anion, on the other hand, is an atom with a negative charge. It has gained an electron or two, or even more. Below are the chemical symbols for three different atoms or atomic anions with 23 electrons.Krypton:Kr has an atomic number of 36, indicating that it has 36 electrons. However, if we add 13 electrons to it, the total number of electrons becomes 49. Krypton with 13 additional electrons becomes Kr-13, with a total of 49 electrons.Chromium:Cr has an atomic number of 24, indicating that it has 24 electrons. Adding two more electrons to it, the total number of electrons becomes 26. The atomic anion with 26 electrons is Cr-2.Oxygen:Oxygen has an atomic number of 8, indicating that it has 8 electrons. However, if we add 15 electrons to it, the total number of electrons becomes 23. Oxygen with 15 additional electrons becomes O-15, with a total of 23 electrons.
for more questions on electrons
https://brainly.com/question/371590
#SPJ8
which of the following describes an experimental technology being used to reduce carbon dioxide emissions from coal?
Answers
Carbon capture and storage is one experimental method being utilised to lower carbon dioxide emissions from coal (CCS).
One experimental technique being used to reduce carbon dioxide emissions from coal is carbon capture and storage (CCS). With CCS, carbon dioxide emissions from factories or power plants are captured and either stored underground in geological formations or used to improve oil recovery.
Coal and other fossil fuels have the potential to drastically cut their carbon dioxide emissions, but CCS technology currently in the experimental stage. Unfortunately, because of its expensive cost and technical implementation difficulties, the technology is not yet extensively employed. In order to address the current climate problem, efforts to cut CO2 emissions are essential.
Learn more about carbon dioxide here:
https://brainly.com/question/9419166
#SPJ4
What experimental technology is being used to reduce carbon dioxide emissions from coal?
Critically discuss the refusal/unwillingness of some individuals to answer questions to put them by authorized Stats SA officials
Answers
People refuse to answer questions from authorized Stats SA officials because they don't want to reveal confidential information.
What does official stats refer to?An official Stats is an official who has the function of asking citizens for different information in order to establish general statistics for all citizens.
Why don't people answer your questions?Some people refuse to answer questions out of mistrust, because they don't want to share personal information.
Learn more about personal information in: https://brainly.com/question/451424
#SPJ1
how many total electron pairs are in the structure of c s 2
Answers
The total number of the electron pairs are eight electron pairs.
What are electron pairs?We know that the structure of a compound can be determined by the number of the electron pairs that are found on the valence shell of the central atom of the compound.
We know that the central atom must be the atom that is least electronegative. Given that there are four valence electrons of carbon which is the central atom in the compound, we have eight electron pairs in the molecule. Thus the total number of valence electrons present is sixteen
Learn more about electron pairs:https://brainly.com/question/19168427
#SPJ1
the constant pressure heat capacity of a sample of a perfect gas was found to vary with pressure according to the expression Cp/(JK⁻¹)=20.17+O.4001T.calculate,q,w,ΔU,Δ H. When the temperature is raised from 0°c to 100°c. At constant pressure and volume
Answers
Answer:
Picture Loading...
___________________________________
Pls brainliest
High-purity benzoic acid (C6H₂COOH; AHxn for combustion = -3227 kJ/mol) is used as a standard
for calibrating bomb calorimeters. A 1.221-g sample burns in a calorimeter (heat capacity = 1365 J/°C)
that contains exactly 1.240 kg of water. What temperature change is observed?
°℃
Answers
According to the given statement 5 °C temperature change is observed.
How does a calorimeter function and what is it?The variation in heat is measured by a calorimeter. A metal water container placed above an exhaust gases serves as the basic component of a calorimeter. The amount of water's temperature change is measured using a thermometer.
Briefing:The calorimeter burns a 1.221g sample.
The calorimeter contains 1.240 kg of water .
The calorimeter has a heat capacity of 1365 j/°C.
∆H rxn = -3227 kj/mol of benzoic acid
Given: 1.221 g is the mass of the benzoic acid
Benzoic acid's molecular weight is 122.12 g/mol.
122.12 g benzoic acid = 1 mol
1.221 g benzoic acid =1.221/122.12= 0.0099 moles
When burned, 1 mol of benzoic acid releases 3227 kj of energy.
Burning 0.0099 moles of benzoic acid produces energy
= 3227kj×(0.0099mols)/1 mol =32.2647 kj =32264.7 j
The calorimeter absorbed thermal energy
= 1365 j/C ×T
Water absorbs heat
= m× C× T
= 1240 g( 4.18 j/gC ) ×T
= 5183.2j/C×T
the total amount of heat absorbed
= 1365T + 5183.2T
=6548.2T
6548.2T=32264.7
T= 4.92 deg.C
T= 5 °C
The result is a 5 °C temperature change.
To know more about Calorimeter visit:
https://brainly.com/question/4802333
#SPJ1
What is the work associated with decomposition of trinitrotoluene (TNT) upon detonation according to the following reaction at 279.65 K ?
C7H5N3O6(s)⟶32N2(g)+52H2(g)+3O2(g)+7C(s)
Answers
The work associated with the decomposition of TNT upon detonation can be determined by using the First Law of Thermodynamics.
What is the first law of thermodynamics?According to the First Law, the change in internal energy (ΔU) of a system is equal to the heat added to the system (q) minus the work done by the system (w).
In this case, the reaction is exothermic and heat is released, meaning that q is negative. The work done by the system can be calculated using the equation:
w = -PΔV
where P is the pressure and ΔV is the change in volume is used to calculate the work done by the system.
Learn more on law of thermodynamics here: https://brainly.com/question/26035962
#SPJ1
electronic configuration of organic compounds
Answers
The electronic configuration of organic compounds depends on the orbitals of their atoms and molecules.
What is electronic configuration?The expression 'electronic configuration' makes reference to the spacial arrangement of electrons in distinct energy orbitals of an atom/molecule.
The orbitals are designed with numbers and letters, whereas the amount of electrons in each orbital is expressed as superscripts (e.g., 1s² 2s² 2p² in the C atom that form glucose).
In conclusion, electronic configuration of organic compounds depends on the orbitals of their atoms and molecules.
Learn more about electronic configurations here:
https://brainly.com/question/26084288
#SPJ1
A class observes two demonstrations water changing into steam and a piece of wood burning and producing smoke. A student concludes
that both demonstrations must be examples of a chemical change because a gas is produced in each.
is the student's conclusion accurate? Explain your answer, referring to both demonstrations.
WILL MARK BRIANLEST
Answers
Answer: The student's conclusion is inaccurate, smoke is a chemical change, but steam is a physical change.
Explanation:
When boiling water changes into steam, the matter composition of water is still the same (it remains H₂O). In this case, only the physical state of the water is changed from a liquid to a vapor (gas). When collected, the water vapor (gas) returns to its original liquid form proving that NO chemical reaction has taken place.
In contrast, a fire is a combustion chemical reaction that converts a fuel material (such as wood, fuel, or paper) and oxygen into carbon dioxide (CO₂) and water (H₂O). As an exothermic chemical reaction, the fire gives off heat in addition to the gaseous smoke. Unlike boiling, the fire permanently alters the fuel material as a result of the chemical reaction process.
Remember to vote for this as Brainliest if I earned it! :)
what happens when muscle cells are triggered?
Answers
Answer:
When myocytes (aka muscle cells) are triggered at a certain point for awhile it can cause strain and pain throughout the muscle.
Express
as
ordinary numbers.
3 x 10^0=
Answers
so 10^0=1 and 3 x 1 = 3 !
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
Calculate the mass of chlorine gas contained in a 5.00dm^3 flask at 25°C and 98KPa pressure
Answers
m/M×RT=PV
Find the mass:
m = (M×P×V)/(R×T)
V = 0.005 m3
T = 298 K
P = 98000 Pa
R = 8.314 J×mol-1×K-1
M = 71 g×mol-1
m = (71×98000×0.005)/(298×8.314) = 14.04 (g)
(Sorry if this didn’t help)
exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. what is the specific heat capacity of the metal
Answers
Exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. The specific heat capacity of the metal is 5.984 J/g°C.
What is specific heat capacity?The heat capacity of a sample of a substance divided by the mass of the sample yields the specific heat capacity (symbol c), also known as massic heat capacity. Informally, it is the quantity of heat that must be added to one unit of a substance's mass in order to raise its temperature by one unit. The specific heat capacity unit in the SI is the joule per kelvin per kilogram, or Jkg⁻¹K⁻¹. For instance, the specific heat capacity of water is 4184 J kg⁻¹K⁻¹, or the amount of energy needed to raise 1 kilogram of water by 1 K.
The specific heat capacity of the metal can be calculated using the equation Q = m × c ×ΔT.
Q = 149.6J
m = 10.0g
ΔT = (final Temperature - initial Temperature) = (25°C - 0°C) = 25°C
Plugging these values into the equation, we get:
149.6J = 10.0g × c ×25°C
Solving for c, we get:
c = \(\frac{149.6J}{(10.0g *25C)}\)
c = 5.984 J/g°C
Therefore, the specific heat capacity of the metal is 5.984 J/g°C.
To know more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ1
3
(2 Points)
How many moles of CO2 is 6.9x1030 particles of CO2?
A. 1.1x10’moles
B. 1.1x10 moles
C.1.3x103 moles
D. 1.3x10’moles
Answers
Answer:
Explanation:
B
Write the structure of nonessential saturated fatty acid with four double bonds and give the name
Answers
The nonessential saturated fatty acid with four double bonds is called Palmitoleic Acid, and its structural formula is CH₃(CH₂)₅CH=CH(CH₂)₇COOH. Its IUPAC name is (Z)-hexadec-9-enoic acid.
What are nonessential saturated fatty acids?Nonessential saturated fatty acids are fatty acids that can be synthesized by the human body and are not required to be obtained from the diet. The human body has the ability to produce these fatty acids through de novo synthesis.
The structure of a nonessential saturated fatty acid with four double bonds is as follows:
Name: Palmitoleic Acid
Structural Formula: CH₃(CH₂)₅CH=CH(CH₂)₇COOH
IUPAC Name: (Z)-hexadec-9-enoic acid
Learn more about nonessential saturated fatty acids on:
https://brainly.com/question/32255805
#SPJ1
A 1,000 kg sports car drives straight at a speed of 200 m/s. How much Kinetic energy does it have? (mass = 1000 kg, speed = 200 m/s).
Answers
Answer: KE =20000000 J
Explanation:
KE=1/2 MV^2
1/2 ( 1000) (200)^2
KE =20000000 J
Which 2 elements has reactivity that is similar to chlorine?
Answers
Convert 1.25 x 1024 atoms of carbon to moles of carbon.
Answers
Answer:
2.076
Explanation:
1 mole is 6.02 * 10^23
To convert from atoms (or molecules or compounds or ions etc.) to mols, you divide the number of atoms (or molecules or etc.) by 6.02 * 10^23
So it is (1.25 * 10^24)/(6.02 * 10^23)
=2.076
Answer:
\(\boxed {\boxed {\sf 2.08 \ mol \ C}}\)
Explanation:
We are asked to convert a number of carbon atoms to moles.
We will use Avogadro's Number for this, which is 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. For this problem, the particles are atoms of carbon. There are 6.022 ×10²³ atoms of carbon in 1 mole of carbon.
We will also use dimensional analysis to solve this problem. To do this, we use ratios. Set up a ratio using the underlined information.
\(\frac {6.022 \times 10^{23} \ atoms \ C}{1 \ mol \ C}\)
We are converting 1.25 ×10²⁴ atoms of carbon to moles, so we multiply the ratio by that value.
\(1.25 \times 10^{24} \ atoms \ C* \frac {6.022 \times 10^{23} \ atoms \ C}{1 \ mol \ C}\)
Flip the ratio. It remains equivalent, but it allows us to cancel the units atoms of carbon.
\(1.25 \times 10^{24} \ atoms \ C* \frac{1 \ mol \ C} {6.022 \times 10^{23} \ atoms \ C}\)
\(1.25 \times 10^{24} * \frac{1 \ mol \ C} {6.022 \times 10^{23} }\)
\(\frac{1.25 \times 10^{24} } {6.022 \times 10^{23} } \ mol \ C\)
\(2.075722351 \ mol \ C\)
The original measurement of atoms has three significant figures, so our answer must have the same. For the number we calculated, that is the hundredths place. The 5 in the thousandths place tells us to round the 7 up to an 8.
\(2.08 \ mol \ C\)
1.25 ×10²⁴ atoms of carbon is equal to approximately 2.08 moles of carbon.
what are levers used for? (science)
Answers
According to the research, levers are used to modify or generate a force and transmit displacement.
What are levers?It is a simple machine composed of a rigid bar of some moderately resistant material, which rotates freely on a support point.
It can be used to maximize the mechanical force applied to an object, increasing its speed or the distance it travels, by applying a proportionally smaller amount of force.
Therefore, we can conclude that according to the research, levers are used to modify or generate a force and transmit displacement.
Learn more about levers here: https://brainly.com/question/19732425
#SPJ1
Cryolite, Na3AlF6(s),
an ore used in the production of aluminum, can be synthesized using aluminum oxide.
equation:
Al2O3(s)+6NaOH(l)+12HF(g)⟶2Na3AlF6+9H2O(g)
If 10.3 kg of Al2O3(s),
55.4 kg of NaOH(l),
and 55.4 kg of HF(g)
react completely, how many kilograms of cryolite will be produced?
mass of cryolite produced:
Answers
The mass (in kilograms) of Cryolite, Na₃AlF₆ produced, given that 10.3 Kg of Al₂O₃, 55.4 Kg of NaOH, and 55.4 Kg of HF react completely is 42.4 Kg
How do i determine the mass of Na₃AlF₆ produced?The mass of Na₃AlF₆ produced from the reaction can be obtained as follow:
Al₂O₃(s) + 6NaOH(l) + 12HF(g) ⟶ 2Na₃AlF₆ + 9H₂O(g
Molar mass of Al₂O₃ = 102 g/molMass of Al₂O₃ from the balanced equation = 1 × 102 = 102 g = 102 / 1000 = 0.102 KgMolar mass of Na₃AlF₆ = 210 g/molMass of Fe from the balanced equation = 2 × 210 = 420 g = 420 / 1000 = 0.420 KgFrom the balanced equation above,
0.102 Kg of Al₂O₃ reacted to produce 0.420 Kg of Na₃AlF₆
Therefore,
10.3 Kg of Al₂O₃ will react to produce = (10.3 × 0.420) / 0.102 = 42.4 Kg of Na₃AlF₆
Thus, from the above calculation, it is evident that the mass of Na₃AlF₆ produced is 42.4 Kg
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
Prove the equilibrium law of pressure kp=kc(RT)^delta n
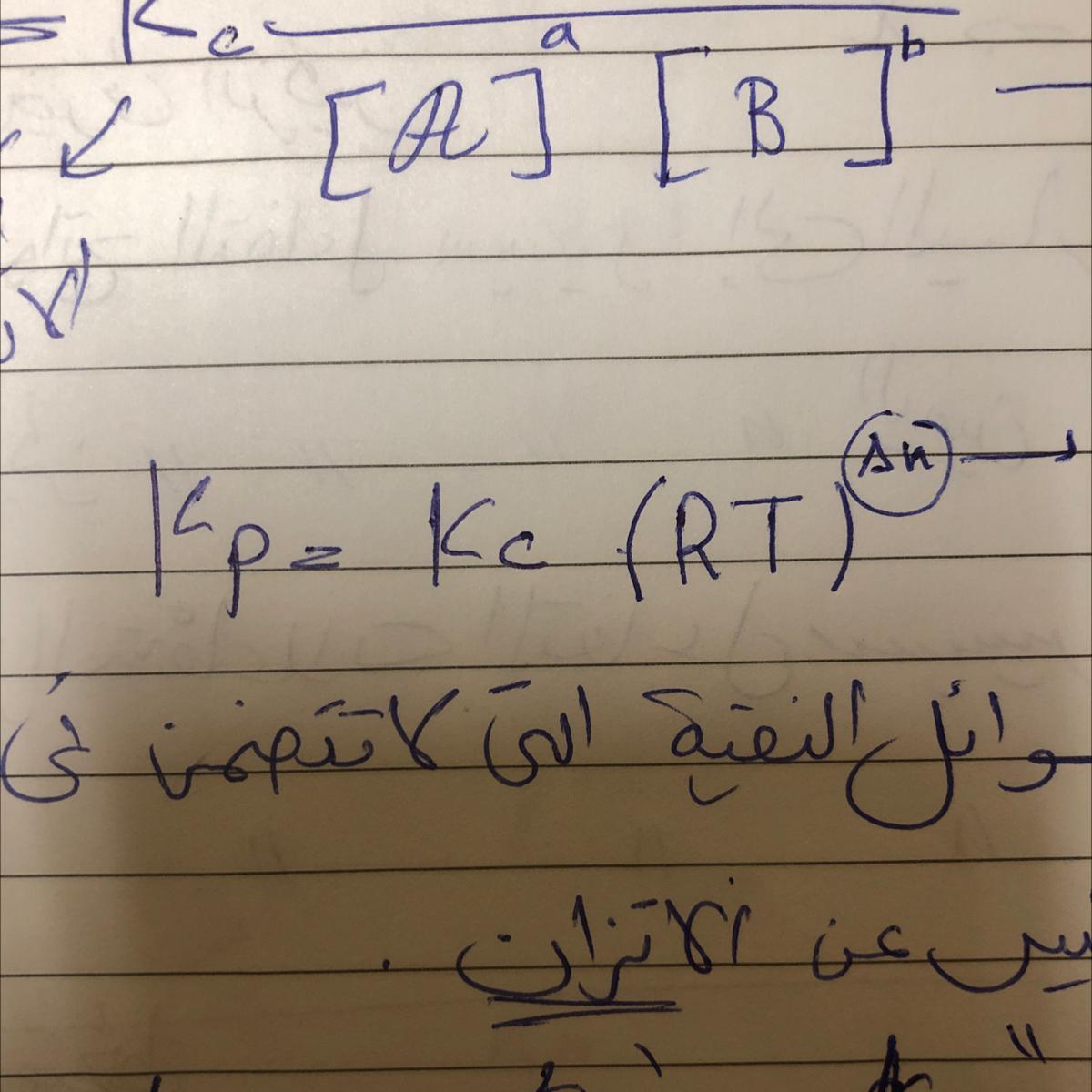
Answers
The solution is in the pictures because the picture is large, so I cut it and also put numbers on it to know it in sequence .
I hope I helped you^_^



At a certain temperature, the equilibrium constant, c,
for this reaction is 53.3.
H2(g)+I2(g)↽−−⇀2HI(g)c=53.3
At this temperature, 0.400 mol H2
and 0.400 mol I2
were placed in a 1.00 L container to react. What concentration of HI
is present at equilibrium?
Answers
At equilibrium, the HI concentration is 2.92 mol/L.
What is the H2 CO2 reaction's equilibrium constant?For the process H2(g)+CO2(g)updownarrow H2O+CO. at 1660 °C, the equilibrium constant KP is 4. In a 5 liter flask, 0.8 moles each of H2 and CO2 are first injected. Reactions occur in the following order with relation to H2: 1. In experiments 1 and 2, the reaction rate doubles when the initial concentration of H2 is doubled while the initial concentration of Cl2 is held constant.
H2(g) + I2(g) ⇌ 2HI(g)
c = [HI]² / [H2][I2]
At the specified temperature, c = 53.3, hence the following can be written:
53.3 = [HI]^2 / (0.400 mol/L) × (0.400 mol/L)
or, [HI]² = 53.3 × 0.16
or, [HI]² = 8.528
or, [HI] = sqrt(8.528) mol/L
or, [HI] = 2.92 mol/L
As a result, 2.92 mol/L of HI are present at equilibrium.
To know more about concentration visit:-
https://brainly.com/question/10725862
#SPJ1
A solution has a [H3O+] 1 x 10^-3 what is the [OH-] of the solution
Answers
Answer:
OH- is 1x 10^ + 3
Explanation:
- and - = +
1. Assume that you have following items and any other necessary equipment.
H₂ gas, O₂ gas, de-ionized water, Li metal, Lit ion-containing proper solution of any
concentration, Zn metal, Zn²+ ion-containing proper solution of any concentration.
(A) Suggest every (theoretically) possible Galvanic cells. The answer must include half-cell
reactions, balanced overall reaction, and Eºcell-
(B) Identify the strongest reducing agent and justify your answer.
(C) If one of the systems suggested in (A) consumes 50g of Zn metal for 2h operation, how
much is the current? How many grams of H₂ can be obtained from water using the
charges of this system?

Answers
(A) Possible Galvanic cells:
Li(s) | Li+ solution || H+ solution | H2(g) | Pt(s)
Half-cell reactions: Li(s) → Li+(aq) + e-; 2H+(aq) + 2e- → H2(g)
Overall reaction: 2Li(s) + 2H+(aq) → 2Li+(aq) + H2(g)
Eºcell = -3.04 V
Zn(s) | Zn2+ solution || H+ solution | H2(g) | Pt(s)
Half-cell reactions: Zn(s) → Zn2+(aq) + 2e-; 2H+(aq) + 2e- → H2(g)
Overall reaction: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
Eºcell = -0.76 V
Zn(s) | Zn2+ solution || O2(g) | H2O(l) | Pt(s)
Half-cell reactions: Zn(s) → Zn2+(aq) + 2e-; O2(g) + 4H+(aq) + 4e- → 2H2O(l)
Overall reaction: Zn(s) + O2(g) + 4H+(aq) → Zn2+(aq) + 2H2O(l)
Eºcell = 1.56 V
(B) The most powerful reducing agent is the one with the lowest Eo value, indicating the greatest proclivity to lose electrons and experience reduction. Based on the Galvanic cells, Li has the most negative Eo value (-3.04 V), making it the most powerful reducing substance.
(c). we can use Faraday's laws of electrolysis to calculate the current and the amount of H₂ gas produced:
Calculate the number of electrons transferred:
From the balanced reaction, we see that 2 moles of electrons (4 e⁻) are transferred for each mole of H₂ produced.
The mass of Zn consumed in 2 hours is 50 g, which is equivalent to 50/65.38 = 0.765 moles of Zn (where 65.38 g/mol is the molar mass of Zn).
Therefore, the total number of electrons transferred is 4 x 0.765 = 3.06 moles of electrons.
Calculate the current:
Faraday's first law states that the amount of chemical change in an electrolytic cell is proportional to the amount of electricity that flows through the cell.
The proportionality constant is the Faraday constant, which is equal to 96,485 C/mol e⁻.
Therefore, the total charge (Q) required to transfer 3.06 moles of electrons is:
Q = 3.06 x 96,485 = 295,038 C
The time (t) taken for this charge to flow through the circuit is 2 hours = 7,200 seconds.
Therefore, the current (I) is:
I = Q/t = 295,038/7,200 = 40.97 A
Calculate the amount of H₂ gas produced:
From the balanced reaction, we know that 1 mole of H₂ gas is produced for every 2 moles of electrons transferred.
Therefore, the number of moles of H₂ gas produced is:
0.765 moles of Zn x (1 mole of H₂/2 moles of electrons) = 0.383 moles of H₂ gas
The molar mass of H₂ is 2 g/mol, so the mass of H₂ gas produced is:
0.383 moles of H₂ gas x 2 g/mol = 0.766 g of H₂ gas
Therefore, the Galvanic cell produces 0.766 g of H₂ gas when it consumes 50 g of Zn metal for 2 hours at a current of 40.97 A.
learn more about Galvanic cells here
https://brainly.com/question/15096829
#SPJ9
please answer fast (i will give 22 points)

Answers
Answer:
red in acid and green in a base
Explanation:
cabbage juice liquid thing is a pH indicator