Answers
IV alkyl halides would afford the indicated product upon reaction with sodium ethoxide.
Alkoxide salt sodium ethoxide is primarily employed as a potent base in organic reactions such deprotonation, dehydration, and dehalogenation. Water and atmospheric carbon dioxide are both likely to react with sodium ethoxide. Even in solid form, this causes samples that have been preserved to degrade with time. Sodium ethoxide samples eventually turn dark when stored, yet the physical appearance of degraded samples may not be noticeable. Ethoxide serves as the counterion in sodium ethoxide, an organic monosodium salt. It performs a function as a nucleophilic reagent.
Which of the following alkyl halides would afford the indicated product upon reaction with sodium ethoxide? I II III or IV?
Learn more about sodium ethoxide here:
https://brainly.com/question/14407408
#SPJ4

Related Questions
PLEASE HELP ASAP
Use what you know about electronegativity to predict the relative strength of the bond between CaO (Calcium Oxide) and
NIO (Nickel II Oxide).
Answers
Answer:
hii
Explanation:
2. A restaurant offers a $19.99 three course meal special that lets you choose an appetizer,
an entree, and a dessert. There are 8 appetizers, 12 entrees, and 6 desserts from which
to choose. How many different meals are available?
Answers
There are 576 different ways to choose a meal.
Promotional offers. A promotional offer is a specific proposition to clients that specifies a reward and patron behavioral standards for earning a reward. the recognition and trouble of the praise are captured thru a retail transaction, purchaser order, rebate claim, rebate redemption, or different patron interplay. a discount is a difference between the unique charge and the lower charge it's far being bought at. a proposal is a deal wherein a product is normally bought at a reduction.
Calculation:-
There are 8 appetizers, 12 entrees, and 6 desserts.
Number of choices = 8 × 12 × 6
= 576
Learn more about different meals here:-https://brainly.com/question/8880115
#SPJ1
Draw the orbital diagram for each of the following metals:
a) [MoCl6]3−
b) [Ni(H2O)6]2+(assume water is weak-field)
c) [MnCl6]4−
Answers
The orbital diagram for each of the following metalsb. The splitting of the is orbitals is smaller in the [Ni(Cl)6]4- complex than in the [Ni(en)3]2+ complex.
What is spectrochemical series?The spectrochemical series is an arrangement of ligands in increasing order of their magnitude of crystal field splitting.
Ligands that occurs towards the right in the series are called strong field ligands and they tend to cause a greater magnitude of crystal field splitting.
Ligands that occur towards the left hand side in the series are called weak field ligands and they tend to cause a lesser magnitude of crystal field splitting.
Therefore, The orbital diagram for each of the following metalsb. The splitting of the is orbitals is smaller in the [Ni(Cl)6]4- complex than in the [Ni(en)3]2+ complex.
Learn more about orbital diagram on:
https://brainly.com/question/28809808
#SPJ1
0.22 L of HNO3 is titrated to equivalence using 0.18 L of 0.2 MNaOH. What is the concentration of the HNO3?
Answers
Answer:
0.16 M
Explanation:
Data provided as per the question is below:-
Volume of \(HNO_3\) = 0.22 L
The Volume of NaOH = 0.18 L
Morality of NaOH = 0.2
According to the given situation, the calculation of the concentration of the \(HNO_3\) is shown below:-
For equivalence,
Number of the equivalent of \(HNO_3\) = Number of equivalents of NaOH
\(= \frac{0.18\times0.2}{0.22}\)
\(= \frac{0.036}{0.22}\)
= 0.16363 M
or
= 0.16 M
The concentration of Fe2 in a sample is determined by measuring the absorbance of its complex with ferroxine. The sample, measured in a 1.00 cm cuvette, has an absorbance of 0.242 . The reagent blank in the same cuvette has an absorbance of 0.041. What would be the absorbance reading for each of these two solutions if measured in a 5.00 cm cuvette
Answers
Answer:
Absorbance of sample solution = 1.21Absorbance of reagent blank = 0.205Explanation:
In order to solve this problem we need to keep in mind the Lambert-Beer law, which states:
A = ε*b*CWhere ε is the molar absorption coefficient, b is the length of the cuvette, and C is the concentration.
By looking at the equation above we can see that if ε and C are constant; and b is 5 times higher (5.00 cm vs 1.00 cm) then the absorbance will be 5 times higher as well:
Absorbance of sample solution = 0.242 * 5 = 1.21Absorbance of reagent blank = 0.041 * 5 = 0.205Consider the reaction below:
2 CO(g) + O₂(g) ⇌ 2 CO₂(g)
If Kc is 2.24 × 10²² at 1273.0 °C, calculate Kp at the same temperature.
Answers
The Kc is 2.8 * 10^24
What is the Kp?In chemistry, Kp usually refers to the equilibrium constant of a reaction that involves gases. It is defined as the ratio of the partial pressures of the products to the partial pressures of the reactants, each raised to the power of their stoichiometric coefficients.
Kp= Kc (RT)^Δn
Thus;
Kc = Kp/(RT)^Δn
Kc = 2.24 × 10²² /(0.082 * 1546)^-1
Kc = 2.8 * 10^24
Thus the Kc of the reaction when we consider the concentration of the reactants is 2.8 * 10^24
Learn more about Kp:https://brainly.com/question/22074421
#SPJ1
The glycerol-3-phosphate shuttle can transport cytosolic NADH equivalents into the mitochondrial matrix (see Fig. 15.11c). In this shuttle, the protons and electrons are donated to FAD, which is reduced to FADH2. These protons and electrons are subsequently donated to coenzyme Q in the electron transport chain. Given that the number of ATP molecules made per NADH and FADH2 oxidation differ by ____? the amount of ATP generated per mole of glucose when the glycerol-3-phosphate shuttle would be ____ instead of 32.
Answers
Answer:
The number of ATP molecules made per NADH and FADH2 oxidation differ by 1.
The amount of ATP generated per mole of glucose when the glycerol-3-phosphate shuttle would be 30 instead of 32.
Explanation:
The glycerol-3-phosphate shuttle can transport cytosolic NADH equivalents into the mitochondrial matrix. In this shuttle, the protons and electrons are donated to FAD, which is reduced to FADH2. These protons and electrons are subsequently donated to coenzyme Q in the electron transport chain. Given that the number of ATP molecules made per NADH and FADH2 oxidation differ by 1, the amount of ATP generated per mole of glucose when the glycerol-3-phosphate shuttle would be 30 instead of 32.
FADH2 generates 1.5 ATP per molecule unlike NADH which generates 2.5 ATP per molecule. This is because electron transfer via FADH2 is not coupled to proton pumping unlike electron transfer reactions involving NADH. Thus, two moles of NADH from the oxidation of 2 moles of glyceraldehyde-2-phosophate to two moles of 1,3-bisphosphoglycerate will yield 3 moles of ATP rather than 5 moles when shuttled through the glycerol-3-phosphate shuttle. The glycerol-3-phosphate shuttle of cytosolic NADH shuttling occurs mainly in the brain and skeletal muscles and does not involve membrane transporters.
3.544g of hydrogen gas contains
Answers
Answer:
1.772 mols
Explanation:
Because hydrogen exists as a diatomic molecule in gaseous state, that's (H2), so then it's molecular mass is 1 ×2= 2g/mol.
we can now calculate for the number of moles, so
n=m/Mr
n=3.544/2
n=1.772mols
Calculate the volume in ml of 0.200 M na2co3 needed to produce 2.00 g of caco3 there is an excess of cacl2
Molar mass of calcium carbonate = 100.09 g/mol
Answers
Answer:
\(V=100mL\)
Explanation:
Hello.
In this case, since the chemical reaction is:
\(Na_2CO_3+CaCl_2\rightarrow CaCO_3+2NaCl\)
We next compute the moles of sodium carbonate from the 2.00 grams of calcium carbonate via their 1:1 mole ratio in the chemical reaction:
\(n_{Na_2CO_3}=2.00gCaCO_3*\frac{1molCaCO_3}{100.09gCaCO_3}*\frac{1molNa_2CO_3}{1molCaCO_3} \\\\n_{Na_2CO_3}=0.0200molNa_2CO_3\)
Thus, by knowing the molarity, we compute the volume:
\(M=\frac{n}{V}\\ \\V=\frac{n}{M}=\frac{0.0200mol}{0.200mol/L}\\ \\V=0.100L*\frac{1000mL}{1L}\\ \\V=100mL\)
Best regards.
The volume in mL of 0.200 M Na₂CO₃ needed to produce 2.00 g of CaCO₃ there is an excess of CaCl₂ is 100mL.
What is molarity?Molarity is define as no. of moles of solute present in per liter of solvent or solution.
Given chemical reaction is:
Na₂CO₃ + CaCl₂ → CaCO₃ + 2NaCl
From the stoichiometry of the reaction it is clear that:
1 mole of CaCO₃ = produced by 1 mole of Na₂CO₃
Moles of CaCO₃ will be calculated as:
n = W/M, where
W = given mass = 2g
M = molar mass = 100.09g/mole
n = 2/100.09 = 0.0200 moles
0.0200 mole of CaCO₃ = produced by 0.0200 moles of Na₂CO₃
Now we calculate the volume by using molarity as:
V = n/M
V = 0.0200/0.2 = 0.1L = 100mL
Hence, the required volume is 100mL.
To know more about molarity, visit the below link:
https://brainly.com/question/16343005
At constant current is passed through an electrolytic cell containing molten MgCl2 for 18 hr. if 4.8 x 105 g of Cl2
are obtained. Calculate the current in Amperes.
Answers
The current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
To calculate the current in amperes, we need to use Faraday's laws of electrolysis and the stoichiometry of the reaction.
Faraday's laws state that the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the cell. The relationship is given by:
Q = nF
Where Q is the electric charge in coulombs (C), n is the number of moles of substance involved in the reaction, and F is Faraday's constant, which is equal to 96,485 C/mol.
In this case, the substance being produced is Cl2, and we know the mass of Cl2 produced, which is 4.8 x 10^5 g.
First, we need to calculate the number of moles of Cl2 produced:
Molar mass of Cl2 = 35.45 g/mol
Moles of Cl2 = mass / molar mass = (4.8 x 10^5 g) / (35.45 g/mol) ≈ 1.354 x 10^4 mol
Now we can calculate the quantity of electricity passed through the cell using Faraday's laws:
Q = nF
Q = (\(1.354 x 10^4\)mol) * (96,485 C/mol)
Q ≈ 1.308 x 10^9 C
The quantity of electricity is given in coulombs. To find the current, we need to divide this value by the time in seconds.
Given that the time is 18 hours, we convert it to seconds:
Time = 18 hours * 60 minutes/hour * 60 seconds/minute
Time = 6.48 x 10^4 seconds
Finally, we can calculate the current:
Current (I) = Q / Time
I = (1.308 x 10^9 C) / (6.48 x 10^4 s)
I ≈ 2.02 x 10^4 Amperes
Therefore, the current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
for more such question on electrolytic visit
https://brainly.com/question/17089766
#SPJ8
If the b of a weak base is 8.1×10−6, what is the pH of a 0.41 M solution of this base?
Answers
The pH of the solution of the weak base here is 11.28.
What is the pH of the solution?
We know that in this case, we have a weak base and we have been asked to obtain the pH of the solution. Let us note that the dissociation of the base would produce the hydroxide ion.
As such we have;
Ka = x^2/0.43 - x
x = concentration of the hydroxide ion
Then;
8.1 * 10^-6 = x^2/0.43 - x
8.1 * 10^-6(0.43 - x) = x^2
3.5 * 10^-6 - 8.1 * 10^-6x = x^2
x^2 + 8.1 * 10^-6x - 3.5 * 10^-6 = 0
x = 0.0019 M
pOH = -log( 0.0019)
= 2.72
pH = 14 - 2.72
= 11.28
Learn more about pH:https://brainly.com/question/15289714
#SPJ1
Alcohol is a
Depressant
Muscle relaxer
Both
Answers
Alcohol is both a Depressant and a Muscle Relaxer. Therefore the answer would be both!
Hope this helps :)
A sample of O2 gas occupies a volume of 571 mL at 26 ºC. If pressure remains constant, what would be the new volume if the temperature changed to:
(a) -5 ºC
(b) 95 ºF
(c) 1095 K
Answers
Answer: The new volume at different given temperatures are as follows.
(a) 109.81 mL
(b) 768.65 mL
(c) 18052.38 mL
Explanation:
Given: \(V_{1}\) = 571 mL, \(T_{1} = 26^{o}C\)
(a) \(T_{2} = 5^{o}C\)
The new volume is calculated as follows.
\(\frac{V_{1}}{T_{1}} = \frac{V_{2}}{T_{2}}\\\frac{571 mL}{26^{o}C} = \frac{V_{2}}{5^{o}C}\\V_{2} = 109.81 mL\)
(b) \(T_{2} = 95^{o}F\)
Convert degree Fahrenheit into degree Cesius as follows.
\((1^{o}F - 32) \times \frac{5}{9} = ^{o}C\\(95^{o}F - 32) \times \frac{5}{9} = 35^{o}C\)
The new volume is calculated as follows.
\(\frac{V_{1}}{T_{1}} = \frac{V_{2}}{T_{2}}\\\frac{571 mL}{26^{o}C} = \frac{V_{2}}{35^{o}C}\\V_{2} = 768.65 mL\)
(c) \(T_{2} = 1095 K = (1095 - 273)^{o}C = 822^{o}C\)
The new volume is calculated as follows.
\(\frac{V_{1}}{T_{1}} = \frac{V_{2}}{T_{2}}\\\frac{571 mL}{26^{o}C} = \frac{V_{2}}{822^{o}C}\\V_{2} = 18052.38 mL\)
Which statement is true according to the kinetic theory?
O A.
OB.
O C.
OD.
Only gases show random motion of particles.
Only gases and liquids show random motion of particles.
Solids have a fixed shape, so they do not show random motion of particles.
All states of matter show random motion of particles.
Reset
Next
Answers
According to kinetic theory of gases, Because solids have a defined geometry, they do not exhibit random particle motion. As a result, choice C is the right one.
A lot of the fundamental ideas of thermodynamics were discovered with the help of the kinetic theory of gases, a straightforward yet historically significant classic model of the thermal behaviour of gases. According to the model, a gas is made up of several identical submicroscopic components (atoms or molecules) that are all moving rapidly and randomly. They are thought to be significantly smaller when measured than the typical particle spacing. Random elastic collisions within the particles and with the container's walls occur between the particles. Because solids have a defined geometry, they do not exhibit random particle motion.
Therefore, the correct option is option C.
To know more about kinetic theory of gases, here:
https://brainly.com/question/15357425
#SPJ1
i am timed please help

Answers
Answer:
mixture
Explanation:
there was leftover components meaning there was something mixed into the liquid
Question:
Seawater contains salt, a/an
molecule that consists of a metal ion and a nonmetal ion.
Answers
Answer:
sodium chloride is an ionic molecule . which is typically the case when metals and nonmetals form bonds
Seawater contains salt, or sodium chloride, which is an ionic compound formed from the bonding of a metal ion (sodium) and a nonmetal ion (chloride) through the transfer of electrons. The ions' opposite charges attract to form the ionic bond.
Explanation:Seawater contains a significant amount of salt, often referred to as sodium chloride. This is a type of ionic compound that consists of a metal ion, sodium (Na), bonded with a nonmetal ion, chloride (Cl). The bond between these ions is formed through the transfer of electrons, resulting in a neutral compound. This is typical of salts, which often consist of a metal and nonmetal ion. The sodium ion carries a positive charge and the chloride ion carries a negative charge, and their attraction forms the ionic bond which holds the salt molecule together in seawater.
Learn more about Salt in Seawater here:https://brainly.com/question/31861244
#SPJ2
Part A
3.75 mol of LiCl in 3.36 L of solution
Express the molarity in moles per liter to three significant figures
Answers
Answer:
1.12 mol/L.
Explanation:
From the question given above, the following data were obtained:
Mole of LiCl = 3.75 moles
Volume = 3.36 L
Molarity =?
Molarity is simply defined as the mole of solute per unit litre of the solution. Mathematically, it is expressed as:
Molarity = mole / Volume
With the above formula, we can obtain the molarity of the solution as follow:
Mole of LiCl = 3.75 moles
Volume = 3.36 L
Molarity =?
Molarity = mole /Volume
Molarity = 3.75 / 3.36
Molarity = 1.12 mol/L
Thus, the molarity of the solution is 1.12 mol/L
Use the mole ratios from the chemical equation to answer the question:
C6H12O6 → 6C + 6H2 + 3O2
How many moles of carbon are produced?
6 moles C
2.5 moles C
30 moles C
15 moles C
Answers
The chemical equation C6H12O6 → 6C + 6H2 + 3O2 states that 6 moles of Carbon (C) are produced when 1 mole of C6H12O6 is reacted. So the correct answer is A)6 moles
This can be determined by using the mole ratios from the equation. The mole ratios show the amount of each substance that is used in the reaction and the amount that is produced. The equation states that for every 1 mole of C6H12O6, 6 moles of Carbon (C) are produced.
To determine the amount of Carbon produced, the mole ratio of Carbon can be used. In this case, the mole ratio is 6:1, meaning that 6 moles of Carbon are produced for every 1 mole of C6H12O6. This means that the answer to the question is 6 moles of Carbon.
In conclusion, the answer to the question "How many moles of carbon are produced?" is 6 moles of Carbon. This answer can be determined by using the mole ratios from the chemical equation C6H12O6 → 6C + 6H2 + 3O2, which states that for every 1 mole of C6H12O6, 6 moles of Carbon are produced. So the correct answer is A)6 moles
for more such questions on Carbon
https://brainly.com/question/27860158
#SPJ11
If you have 50 grams of H2SO4 and excess
Al, how many grams of Al2(SO4)3 are
produced during the following reaction?
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2
Answers
0.65mol \(Alx_{2} (So_{4} )_{3}\) are produced during the reaction.
What is the molar mass?The mass in grams of one mole of a chemical is its molar mass. A mole is the measurement of the number of things, such as atoms, molecules, and ions, that are present in a substance. Any substance's mole is. Molecules, 6.022 10 23.Divide each element's atomic weight (found in the periodic table) by the number of that element's atoms in the compound. 3. Add up the totals, and then follow the number with the units of grams/mole.The mass of every atom in a molecule, expressed in grams per mole, is known as the molar mass. We first obtain the atomic weights of the different elements in a periodic table to compute a molecule's molar mass. Then, we multiply the total number of atoms by each one's atomic mass.How many grams of Al2(SO4)3 are produced during the following reaction?
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2
Molar mas:26.98 →341.15g/mol
All take 35g.
Moles of aluminum=35/26.98=1.29mol.
2mole of aluminum will give=1/2*1.29=0.65
Therefore, 0.65mol \(Alx_{2} (So_{4} )_{3}\).
To learn more about Molar mass, refer to:
https://brainly.com/question/837939
#SPJ9
Please help meeeeeee ? what is the answer

Answers
Answer:
D
Explanation:
In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between.
cos(A)+cos(2A)+cos(3A)=0 is not an identity
Answers
Answer:
this is the required answer look it once

The greatest ________ in human population occurs during the last few centuries
Answers
describe the trend in electronegativity of the elements ?
Answers
Answer:
On the periodic table, electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. As a result, the most electronegative elements are found on the top right of the periodic table, while the least electronegative elements are found on the bottom left.
Explanation:
Make sure to edit so you don't get copy-writed.
Which statement is one of the assumptions of Kinetic Molecular Theory regarding gasses?
A Collisions between gas particles are inelastic.
The temperature of the gas depends on the average potential energy of the gas particles.
Gas particles are much larger than the distance between them.
The volume of a gas sample mostly consists of empty space between the moving gas particles.
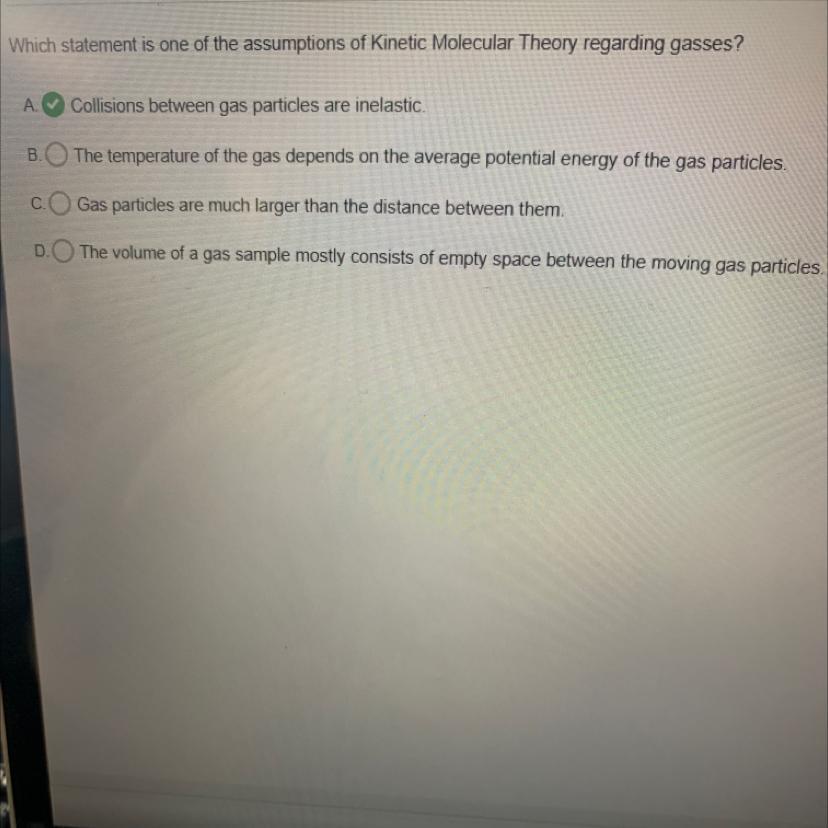
Answers
The statement that "The volume of a gas sample mostly consists of empty space between the moving gas particles" is one of the assumptions of the Kinetic Molecular Theory regarding gases.
What is Collision?
There are different types of collisions, depending on the nature of the objects involved, the speed and direction of their motion, and the type of contact that occurs. For example, elastic collisions are those in which the total kinetic energy of the colliding objects is conserved, meaning that no energy is lost or gained during the collision. In contrast, inelastic collisions are those in which some of the kinetic energy is transformed into other forms of energy, such as heat or sound.
The Kinetic Molecular Theory is a model that describes the behavior of gases. One of the main assumptions of this theory is that gas particles are in constant random motion and move in a straight line until they collide with other particles or the walls of their container.
Another important assumption of this theory is that the volume of a gas sample mostly consists of empty space between the moving gas particles. This means that gas particles are assumed to be very small compared to the overall volume of the gas sample. Therefore, the particles do not occupy all of the available space in the container, but instead only occupy a small portion of it.
Learn more about Collision from given link
https://brainly.com/question/24915434
#SPJ1
The big bang theory states that the Universe began by expanding from a small point nearly 14 billion years ago. Since that time, the Universe has continued to expand, and it is still expanding today.
In 1964, two scientists, Arno Penzias and Robert Wilson, were studying electromagnetic radiation emitted from space. During their research, they detected background radiation coming from all directions in the sky. This background radiation was found to be microwaves created during the big bang.
What does this information demonstrate?
A.
Most scientific theories of the Universe's origin remain the same even if scientific data fails to support them.
B.
Scientific data can be used to help support a theory of the origin of the Universe.
C.
The origin of the Universe should be explained by stating a theory is correct before collecting data.
D.
Scientific data can be used to help show that theories of the Universe's origin should always remain the same.
Answers
The information provided demonstrates that Option B. Scientific data can be used to help support a theory of the origin of the Universe.
The discovery of background radiation coming from all directions in the sky by Penzias and Wilson supports the big bang theory, which proposes that the Universe began expanding from a small point nearly 14 billion years ago. This discovery of cosmic microwave background radiation was predicted by the big bang theory, providing strong evidence in favor of the theory.
This example also highlights the importance of empirical evidence in scientific theories. The big bang theory was proposed based on observations of the redshift of distant galaxies, and the discovery of the cosmic microwave background radiation provided further empirical support for the theory. As more data is collected and analyzed, scientific theories can be modified or replaced if the evidence does not support them. This is the essence of the scientific method, which involves formulating hypotheses based on observations and then testing them through experimentation and observation.
Therefore, scientific data plays a crucial role in supporting or disproving theories, and theories should be continually tested and modified based on new evidence. The big bang theory remains one of the most widely accepted explanations of the origin of the Universe, and it is continually being refined and modified as new data becomes available. So, Option B is Correct.
Know more about the Big bang theory here :
https://brainly.com/question/31377991
#SPJ11
Help pls i really need help

Answers
Answer: I think it's 1
Explanation: Potential energy means stored energy . In Position 1 it's not moving the energy is being stored. Hope that helps.
What type of reaction is KBr + Cl2 => KCl + Br2?
Answers
Answer:
displacement reaction
Explanation:
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
6. How many ions of Na are in 45.6 g of Na2SO4?
Answers
Answer:
3.85 ×10²³ Na⁺ ions
Explanation:
Given data:
Mass of Na₂SO₄ = 45.6 g
Number of ions of Na = ?
Solution:
Number of moles of Na₂SO₄:
Number of moles = mass/ molar mass
Number of moles = 45.6 g/ 142.04 g/mol
Number of moles = 0.32 mol
1 mole of Na₂SO₄ contain 2 moles of Na⁺ ion.
0.32 mol × 2= 0.64 moles of Na⁺ ion
1 mole contain 6.022×10²³ Na⁺ ions
0.64 mol × 6.022×10²³ Na⁺ ions / 1 mol
3.85 ×10²³ Na⁺ ions
Who made the first periodic table
Answers
The correct answer is Dmitri Mendeleev