When a 1.00-g sample of methane gas was burned with excess oxygen in the calorimeter, the temperature increased by 7.3°C. When
a 1.00-g sample of hydrogen gas was burned with excess oxygen, the temperature increase was 14.3°C. Compare the energies of
combustion (per gram) for hydrogen and methane.
a. 10kJ CH4, 100kJ H2.
b. 160kJ CH4, 80kJ H2
c. 80kJ CH4, 160kJ H2-
d. 100kJ CH4, 10k) H2
Answers
Answer:
The energies of combustion (per gram) for hydrogen and methane are as follows: Methane = 82.5 kJ/g; Hydrogen = 162 kJ/g
Note: The question is incomplete. The complete question is given below:
To compare the energies of combustion of these fuels, the following experiment was carried out using a bomb calorimeter with a heat capacity of 11.3 kJ/℃. When a 1.00-g sample of methane gas burned with
excess oxygen in the calorimeter, the temperature increased by 7.3℃. When a 1.00 g sample of hydrogen gas was burned with excess oxygen, the temperature increase was 14.3°C. Compare the energies of combustion (per gram) for hydrogen and methane.
Explanation:
From the equation of the first law of thermodynamics, ΔU = Q + W
Since there is no expansion work in the bomb calorimeter, ΔU = Q
But Q = CΔT
where C is heat capacity of the bomb calorimeter = 11.3 kJ/ºC; ΔT = temperature change
For combustion of methane gas:
Q per gram = ( 11.3 kJ/ºC * 7.3°C)/1.0g
Q = 83 kJ/g
For combustion of hydrogen gas:
Q per gram = ( 11.3 kJ/ºC * 14.3°C)/1.0g
Q = 162 kJ/g
Related Questions
which of the following are compounds? select all that apply.? a) FeCl3
b) Mg
c) P8
d) Fe
e) N2O5
f) CH4
g) F2
h) CuO
Answers
FeCl3, CH4, \(F_2\), and CuO are compounds. Thus option A, F, G, H are the answers.
A compound is a substance made up of two or more different elements that are chemically bonded together. The chemical formula for a compound represents the ratio of the elements present in the compound.
a) FeCl3 is a compound, which is made up of Iron and Chlorine elements chemically bonded together. The chemical formula for FeCl3 is FeCl3, representing the ratio of Iron and Chlorine in the compound.
f) CH4 is a compound, which is made up of Carbon and Hydrogen elements chemically bonded together. The chemical formula for CH4 is CH4, representing the ratio of Carbon and Hydrogen in the compound.
g) F2 is a compound, which is made up of Fluorine element chemically bonded together. The chemical formula for F2 is F2, representing the ratio of Fluorine in the compound.
h) CuO is a compound, which is made up of Copper and Oxygen elements chemically bonded together. The chemical formula for CuO is CuO, representing the ratio of Copper and Oxygen in the compound.
On the other hand, b) Mg is an element, not a compound. It represents Magnesium, which is a single element and not made up of two or more different elements. Similarly, c) P8 is not a chemical formula, so it is not a compound. It represents an unknown compound. d) Fe is an element, not a compound. It represents Iron, which is a single element. e) N2O5 is a molecule, not a compound. It represents Dinitrogen pentoxide, which is made up of Nitrogen and Oxygen elements but not chemically bonded together.
Learn more about Compounds:
https://brainly.com/question/25982116
#SPJ4
mass of 1×10^25 molecules of water
Answers
Answer:
1.E25 it is the answer the answer to mass of 1×10^25 molecules of water
Explanation:
this is just EXPLINATION find your answer using this
first divide the number of molecules by Avogadro's number 6.022*10^25
you will
l get no. of Moles of water
multiply the no. of Moles with mass of 1 Mole of water 18g per mole
if get answer you comment
you should try on your own you will understand better
HELP IM CONFUSED I GIVE BRAINLIEST

Answers
Answer:
I⁻ , Sr²⁺ , K⁺
Explanation:
refer to the periodic table and memorize trends in charges.
Describe how to calculate the Rf value for a spot on a TLC plate.
Answers
Answer:
In thin-layer chromatography, the retention factor (Rf) is used to compare and help identify compounds. The Rf value of a compound is equal to the distance traveled by the compound divided by the distance traveled by the solvent front (both measured from the origin).
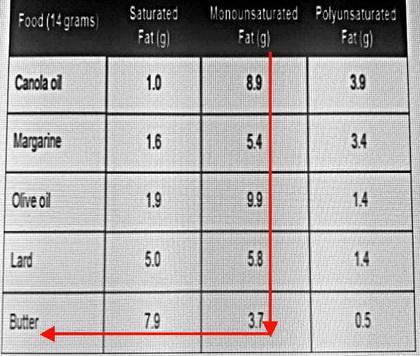
Which food has the least amount of monounsaturated fat?
Answers
Answer:
Butter
Explanation:
Assume you have a table like the one below.
It tells you the mass of each type of fat in a 14 g sample of food.
Start at the top of the Monounsaturated Fat column.
Go straight down until you reach the smallest number in the column (3.7).
Then, move horizontally left until you reach the Food column.
The food with the least amount of monounsaturated fat is butter.
What does it mean for an object to be dense?
Answers
Answer: Density is a measure
Explanation: The more squashed together an object’s particles are, the denser it is.
Who did the ram caught in the thicket (Genesis 22:13) represent?
Answers
Answer:
“Rams Caught in a Thicket” as an allusion to the biblical story of Abraham. They more likely represent goats, which could often be seen standing on their hind legs, to reach leaves in the trees, as seen here.
An organic compound (CaHbNcOdCle) was synthesized and a sample of it was analyzed and found to contain only C, H, N, O, and Cl. It was observed that when a 0.150-g sample of the compound was burned, it produced 0.138 g CO2 and 0.0566 g H2O. All the nitrogen in a different 0.200-g sample of the compound was converted to NH3, which found to weigh 0.0238 g. Finally, the chlorine in a 0.125-g sample of the compound was converted to Cl- and by reacting it with AgNO3, all the chlorine was recovered as AgCl. The AgCl, when dried, was found to weigh 0.251 g. Calculate the weight percent of each element in the compound.
Answers
The mass percent of each element is:
mass percent of C is 24%
mass percent of H is 4%
mass percent of Cl is 50%
mass percent of N is 9.8%
Mass percent of oxygen is 12.2%
What is the mass of each of the constituent elements in a sample of the compound?The mass of each of the constituent elements in a sample of the compound is determined as follows:
mass of C:
1 mole of C is present in 1 mole of CO₂
The mass of C in 0.138 g of CO₂ will be:
0.138 g / 44 g * 12 g = 0.0368 g
mass of H:
2 moles of H are present in 1 mole of H₂O
The mass of H present in 0.0566 g H₂O will be:
0.0566 g / 18 * 2 * 1 = 0.00628 g
mass of Cl;
1 mole of Cl is present in 1 mole of AgCl
The mass of Cl in 0.251 g of AgCl will be:
0.251 g / 143.5 g * 35.5 g = 0.0621 g
mass of N:
1 mole of N is present in 1 mole of NH₃
The mass of N in 0.251 g of NH₃ will be:
0.0238g / 17.0 g * 14 g = 0.0196 g
Mass percent of each element will be:
mass percent of C:
mass percent = 0.0368/0.15 * 100
mass percent = 24%
mass percent of H:
mass percent = 0.00628 / 0.15 * 100%
mass percent = 4%
mass percent of Cl;
mass percent of Cl:
mass percent = 0.0621 / 0.125 * 100%
mass percent = 50%
mass percent of N:
mass percent = 0.0196 / 0.2 * 100%
mass percent = 9.8%
Mass percent of oxygen = 100 - (24 + 4 + 50 + 9.8)
Mass percent of oxygen = 12.2%
Learn more about mass percent at: https://brainly.com/question/29565632
#SPJ1
Select the statement which explains the meaning of the symbol 6d5.
A. The principal quantum number (n) is 6, the angular momentum quantum number (ell) is 5, and there are 2 electrons in the subshell.
B. The principal quantum number (n) is 6, the angular momentum quantum number (ell) is 2, and there are 5 electrons in the subshell.
C. The principal quantum number (n) is 5, the angular momentum quantum number (ell) is 5, and there are 5 electrons in the subshell.
D. The principal quantum number (n) is 5, the angular momentum quantum number (ell) is 4, and there are 4 electrons in the subshell.
Answers
Answer:
B. The principal quantum number (n) is 6, the angular momentum quantum number (ell) is 2, and there are 5 electrons in the subshell.
Explanation:
Given;
electronic configuration of 6d⁵
where;
6 in the configuration shows that, the principal quantum number (n) is 6
d is the subshell
d orbital has angular momentum quantum number (l) of 2
s orbital, l = 0
p orbital, l = 1
d orbital, l = 2
f orbital, l = 3
5 in the configuration means that there are 5 electrons in the subshell
Therefore, 6d⁵ symbol means that "The principal quantum number (n) is 6, the angular momentum quantum number (ell) is 2, and there are 5 electrons in the subshell".
a. Un estereoisómero del 1,1,3,5-tetrametilciclohexano es 15 kJ/mol (3.7 kcal/mol) menos estable que el otro. Indicar cuál isómero es el menos estable e identificar la razón de la disminución de la estabilidad.
Answers
The cis isomer of 1, 1, 3, 5-tetramethylcyclohexane is more stable than the trans of 1, 1, 3, 5-tetramethylcyclohexane. This also implies that former has a higher stability and energy than the later.
Stable 1, 1, 3, 5-tetramethylcyclohexaneThe picture attached to this answer sheet is cis isomer of 1, 1, 3, 5-tetramethylcyclohexane
Also even, the cis isomer of 1, 1, 3, 5-tetramethylcyclohexaneexist in two forms
Diaxial formDiequatorial formLearn more about organic compounds:
https://brainly.com/question/704297

Phosphoric acid, H3PO4, will undergo three successive ionization reactions to varying extents in water. What is the balanced equilibrium identified as Ka3
Answers
Answer:
HPO₄⁻² + H₂O ⇄ PO₄⁻³ + H₃O⁺ Ka3
Explanation:
Phosphoric acid, H3PO4 is a dyprotic acid which undergoes in these three succesive ionization reactions.
The last equillibrium is conditionated to the Ka3
H₃PO₄ + H₂O ⇄ H₂PO₄⁻ + H₃O⁺ Ka1
H₂PO₄⁻ + H₂O ⇄ HPO₄⁻² + H₃O⁺ Ka2
HPO₄⁻² + H₂O ⇄ PO₄⁻³ + H₃O⁺ Ka3
It is an acid because it release a proton, to make the [H₃O⁺] rise.
pH of solution will always be < 7
H₂PO₄⁻ and HPO₄⁻² are amphoteric compounds, which means that they can work as an acid (release protons), or base (take protons)
Why do thermistors increase in conductivity when heated? What happens in normal metals? Explain on the atomic level.
Answers
Metal conductivity generally goes down or resistivity goes up with temperature goes up.
why specific gravity test is done ?
Answers
Answer: Urinary specific gravity (SG) is a measure of the concentration of solutes in the urine. It measures the ratio of urine density compared with water density and provides information on the kidney's ability to concentrate urine. A urinary specific gravity measurement is a routine part of urinalysis. A urine specific gravity test compares the density of urine to the density of water. This quick test can help determine how well your kidneys are diluting your urine. Urine that's too concentrated could mean that your kidneys aren't functioning properly or that you aren't drinking enough water.
Hope this helps..... Stay safe and have a Merry Christmas!!!!!!!!!! :D
Explanation:
How many moles (of molecules or formula units) are in each sample?
17.0 g NO2
Express your answer in moles to three significant figures.
Answers
Answer:
4 molecules and if there will ever happen if I don't 6b you to be able I can ask for a better price for you
52.30 ounces = how many milliliters use correct significant figures
Answers
Answer:
1547 mL
Explanation:
Use the following conversion factor for ounces to mL:
1 ounce = 29.5735 mL
Set up the equation like this:
\(52.30oz *\frac{29.5735 mL}{1 oz}\)
Solve:
Using a calculator we get 1546.696 mL.
There are 4 sig figs in 52.30, so the correct answer would be 1547 mL.
Hope this helps!!!
A heated sample was found to contain
85.25 g of anhydrous compound and
14.75 g H₂O. The molar mass of the
anhydrate is 208 g/mol. What is the value
of "n" in the hydrate formula?
A. 15
B. 1
C. 2
D. 10
Anhydrous Compound - nH₂O
.
Answers
This means that the ratio of anhydrous compound to water in the hydrate is 1:2. Therefore, the value of "n" in the hydrate formula is 2.
What is a hydrate and how to find ?To determine the value of "n" in the hydrate formula, we need to use the given information to calculate the number of moles of anhydrous compound and water in the sample, and then use the mole ratio between them to determine the value of "n".
First, we can calculate the number of moles of anhydrous compound:
moles of anhydrous compound = mass / molar mass
moles of anhydrous compound = 85.25 g / 208 g/mol
moles of anhydrous compound = 0.4091 mol
Next, we can calculate the number of moles of water:
moles of water = mass / molar mass
moles of water = 14.75 g / 18.015 g/mol
moles of water = 0.8180 mol
Anhydrous Compound - nH₂O
The mole ratio between the anhydrous compound and water is:
moles of anhydrous compound : moles of water
0.4091 mol : 0.8180 mol
We can simplify this ratio by dividing both sides by the smaller value:
0.4091 mol / 0.4091 mol : 0.8180 mol / 0.4091 mol
1 : 2
To know more about anhydrous compound visit:-
brainly.com/question/27078278
#SPJ1
which type of alcohol undergo oxidation under normal conditions
Answers
Answer:
Primary alcohols can be oxidized to form aldehydes and carboxylic acids; secondary alcohols can be oxidized to give ketones. Tertiary alcohols, in contrast, cannot be oxidized without breaking the molecule's C–C bonds.
Based on the following equation:
3 H₂ + N₂ - 2NH3
If 3.24 moles of ammonia gas are produced, how many moles of hydrogen gas were consumed in the reaction?
Answers
The process used 4.86 moles of hydrogen gas to generate 3.24 moles of ammonia gas.
How to determine moles consumed?According to the balanced chemical equation, the stoichiometry of the reaction shows that 3 moles of hydrogen gas (H₂) react with 1 mole of nitrogen gas (N₂) to produce 2 moles of ammonia gas (NH₃).
So, for every 2 moles of NH₃ produced, we need 3 moles of H₂ consumed. Therefore, to determine the moles of H₂ consumed, set up a proportion:
3 moles H₂ / 2 moles NH₃ = x moles H₂ / 3.24 moles NH₃
where x is the number of moles of H₂ consumed.
Solving for x:
x = (3 moles H₂ / 2 moles NH₃) x (3.24 moles NH₃) = 4.86 moles H₂
Therefore, 4.86 moles of hydrogen gas were consumed in the reaction to produce 3.24 moles of ammonia gas.
Find out more on hydrogen gas here: https://brainly.com/question/19813237
#SPJ1
Question 4 of 10
Which of the following is the correct model of C.H14?
o
A.
O
B.
O C.
D.

Answers
Answer:
B
Explanation:
Option B is correct because you have 6 vertices, indicating that there ate 6 carbons in the compound.
You react 4.16g of sodium hydroxide with 5.81g hydrochloric acid according to the following reaction you produce 5.79 g of sodium chloride. What is your percent yield?
Answers
The limiting reactant in the reaction is NaOH. one mole or 40 g of NaOH gives 58.5 g of NaCl. Thus, 4.16 g have to give 6.08 g but the actual yield is 5.79 g. Thus the percent yield of the reaction is 95%.
What is percent yield?Percent yield of a reaction is the ratio of actual yield to the theoretical yield multiplied by 100.
The molar mass of NaOH is 40 g/mol. Thus number of moles of NaOH is 4.16 /40 = 0.10 moles. Similarly the molar mass of HCl is 36.5 g/mol and number of moles in 5.81 g is 5.81/ 36.5 = 0.15 moles.
One mole of HCl needs 1 mole of NaOH, thus 0.15 moles need 0.15 moles NaOH but we have 0.1 only. Thus, NaOH is the limiting reactant.
40 g of NaOH gives 58.5 g of NaCl (1 mole). Thus the theoretical yield for 4.16 g of NaOH is :
= ( 4.16 × 58.5 )/40
= 6.04 g.
The actual yield is 5.79 g. Thus percent yield =(5.79/6.04) × 100 = 95%.
Hence, the percent yield of the reaction is 95%.
To find more on percent yield , refer here:
https://brainly.com/question/12704041
#SPJ1
In this experiment we will be using a 0.050 M solution of HCl to determine the concentration of hydroxide (OH-) in a saturated solution of calcium hydroxide. The initial volume of "filtered" calcium hydroxide solution used in this example will be 36.0 mL. If it takes 16 mL of the HCl solution to reach the equivalence point of the titration, how many moles of OH- were present in this sample of calcium hydroxide?
How many moles of Ca2+ ?
Recall, the values used to determine Ksp values are concentrations. Considering the initial volume of calcium hydroxide was 36 ml, the concentration of OH- is
, and the concentration of Ca2+ is
Using these values, what is the Ksp of calcium hydroxide according to this data?
Report all your answers in scientific notation.
Answers
Based on the definition of equivalence point and the data provided;
the moles of OH- present is 0.0008 molesthe moles of Ca2+ present is 0.0004 moles.The Ksp of calcium hydroxide 5.324 × 10^-8What is equivalence point of a reaction?The equivalence point of a reaction is the point at which equal amounts of both acid and base have reacted.
Equation of the reaction is as follows:
HCl + Ca(OH)2 ---> CaCl2 + 2 H20
Moles of HCl reacted = molarity × volume in L
Moles of HCl reacted = 0.05 M × 16/1000 = 0.0008 moles
Therefore, moles of OH- present = 0.0008 moles
Also, moles of Ca2+ present = 0.0008/2 = 0.0004 moles
The molar solubility product Ksp of calcium hydroxide is calculated as follows.
Ksp = [Ca2+]^2 × 2 × [OH]volume of solution = 36 mL = 0.036 L
[Ca2+] = 0.0004 / 0.036 = 0.011 M
[OH] = 0.0008 / 0.036 = 0.022 M
Ksp = (0.011)^2 × 2(0.022)
Ksp = 5.324 × 10^-8
Therefore, the moles of OH- present is 0.0008 moles and the moles of Ca2+ present is 0.0004 moles.
The Ksp of calcium hydroxide 5.324 × 10^-8
Learn more about equivalence point at: https://brainly.com/question/24584140
22.55 mL of an H2SO4 solution
were titrated with 14.85 mL of a
0.146 M NaOH solution to reach the
equivalence point. What is the
molarity of the H2SO4 solution?

Answers
The concentration of H₂SO₄ solution is equal to 0.0480 M.
What is a neutralization reaction?A neutralization reaction is described as a chemical reaction where acid and base react to produce respective salt and water. When a strong acid reacts with a strong base then the salt can be neutral.
When H₂SO₄ (a strong acid) reacts with NaOH, the resulting salt is Na₂SO₃ and water.
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2H₂O
Given, the concentration of NaOH = 0.146 M
The volume of the NaOH = 14.85 ml = 0.01485 L
The number of moles of NaOH, n = M × V = 0.146 × 0.01485 = 0.00216 M
The volume of the H₂SO₄ = 22.55 ml = 0.02255 L
The number of moles of H₂SO₄, n = 0.00216/2 = 0.00108 mol
The concentration of H₂SO₄ =0.00108/0.02255 = 0.0480 M
Therefore, the molarity of H₂SO₄ is 0.0480 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
Exoplanets are usually....
O big, bright and close to the sun.
O gigantic, fireballs that are close to the sun.
O not a planet.
O small, dark, and far from the sun.
Answers
Exoplanets are usually gigantic, fireballs that are close to the sun. Therefore, the correct option is option B.
Any planet outside of our solar system is an exoplanet. The majority of exoplanets orbit other stars, while rogue planets—free-floating exoplanets that are unattached to any star—orbit the galactic centre.
The majority of the exoplanets found so far are in the Milky Way, which is a rather tiny area of our galaxy. The Kepler Space Telescope of NASA has revealed that the galaxy has more planets than stars. Exoplanets are usually gigantic, fireballs that are close to the sun.
Therefore, the correct option is option B.
To know more about exoplanets, here:
https://brainly.com/question/29837455
#SPJ1
Why doesn't the red line showing the IR spectrum emitted from the earth's surface match the blue line showing the expected IR spectrum from a 300˚C object?
-Some of the light emitted is used to heat building.
-Molecules in the atmosphere such as CO2 and H2O absorb the radiation.
-Contrails from airplanes absorb the radiation cause the dip at 14 micrometers.
-IR radiation at 14 micrometers is not actually emitted by the earth's surface.
Answers
The red line showing the IR spectrum emitted from the earth's surface does not match the blue line showing the expected IR spectrum from a 300˚C object because:
Molecules in the atmosphere such as CO₂ and H₂O absorb the radiation; option B.What is IR spectroscopy?IR spectroscopy studies Infrared (IR) light in the electromagnetic spectrum.
Sensors are used by thermal detection systems, also known as infrared detection systems, to detect radiation in the infrared region of the electromagnetic spectrum.
In order to create an electronic signal, an infrared camera must first detect the thermal energy or heat, that the scene being seen emits. After processing this signal, an image is created.
Learn more about IR spectroscopy at: https://brainly.com/question/29493769
#SPJ1
Iron and Chlorine gas react according to the following balanced equation: 2 Fe(S) + 3 Cl2 (g) 2 FeCl3(s) a) Calculate the molar mass in grams of “one mole” of each of the following: Fe ________ Cl2 __________________ FeCl3 ______________
Answers
The molar mass in grams of "one mole" of each substance is:
Fe: 55.845 g/mol
\(Cl_2\): 70.906 g/mol
\(FeCl_3\): 162.204 g/mol
To calculate the molar mass in grams of "one mole" of each substance, we need to determine the atomic masses of the elements involved in the equation.
The atomic mass of iron (Fe) is 55.845 g/mol.
For chlorine (\(Cl_2\)), we need to consider that the molar mass of \(Cl_2\) is twice the atomic mass of chlorine because the formula shows that two chlorine atoms combine to form one molecule of \(Cl_2\). The atomic mass of chlorine is 35.453 g/mol, so the molar mass of \(Cl_2\) is 2 * 35.453 g/mol = 70.906 g/mol.
The formula for iron(III) chloride (\(FeCl_3\)) indicates that one mole of \(FeCl_3\)contains one mole of iron and three moles of chlorine. Therefore, we can calculate the molar mass of \(FeCl_3\)by summing the atomic masses of iron and chlorine:
Molar mass of \(FeCl_3\)= (1 * atomic mass of Fe) + (3 * atomic mass of Cl)
Substituting the values, we have:
Molar mass of \(FeCl_3\) = (1 * 55.845 g/mol) + (3 * 35.453 g/mol)
= 55.845 g/mol + 106.359 g/mol
= 162.204 g/mol
For more such questions on molar mass visit:
https://brainly.com/question/837939
#SPJ8
A mixture of 0.384 M H2O, 0.384 M Cl2O, and 0.652 M HClO are placed in a vessel at 25 oC. Calculate the equilibrium concentration (in molarity) of HClO at the same temperature.
Answers
Equilibrium concentration of HOCl at 25°C is 0.140 M
The equation of the reaction at equilibrium is given below:
H₂O (g) + Cl₂O (g) ⇄ 2 HOCl (g)
Equilibrium constant, Kc = 0.0900 at 25°C
An ICE chart for the reaction is set up below
H₂O (g) + Cl₂O (g) ⇄ 2 HOCl (g)
I 0.384 0.384 0.652
C -x -x + 2x
E 0.384 - x 0.384 - x 0.652 + 2x
The equation of the equilibrium constant is given as;
Kc = \(\frac{[HOCl]^2}{[H_{2}O][Cl_{2}O]}\)
substituting the values into the equation:
Kc = \(\frac{(0.652 + 2x)^2}{( 0.384 - x)( 0.384 - x)}\) = 0.0900
4x² + 2.608x + 0.425 = 0.0900 (0.147 - 0.768x + x²)
4x² - 0.09x² + 2.677x - 0.069x + 0.425 - 0.013 = 0
3.910x² + 2.677x + 0.412 = 0
Solving using the quadratic formula:
a = 3.910, b = 2.677, c = 0.412
x = \(\frac{-2.677\±\sqrt{2.677^2 - 4*3.910*0.412}}{2*3.910}\)
x = -0.256 or -0.431
The negative sign indicates that equilibrium is to the left of the reaction
Since x cannot be greater than 0.384, x = -0.256.
Equilibrium concentration of HOCl = 0.652 + 2(-0.256)
Equilibrium concentration of HOCl at 25°C is 0.140 M
Learn more at: https://brainly.com/question/15050697
A sample of 2 moles of Neon is in a 1.5 L container at STP ( 0C and 101.3kpa). If the volume is doubled, what would have to happen to the number of moles of Ne to maintain STP? calculate how many moles of Ne would be needed
Avogadro's law
Answers
Answer:
According to Avogadro’s law, at a constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas. This means that if the volume of the container is doubled while maintaining STP (standard temperature and pressure), the number of moles of Ne would also have to double in order to maintain STP.
Since the initial number of moles of Ne is 2, if the volume is doubled, the number of moles of Ne would have to increase to 2 * 2 = 4 moles to maintain STP.
If the volume is doubled, the number of moles of Neon would have to be doubled as well to maintain STP conditions.
The initial volume of Neon is 1.5 L, which contains 2 moles of Neon. If the volume is doubled, the new volume is 3 L, and we need to calculate how many moles of Neon would be needed to maintain STP.
Using the ideal gas law, PV = nRT, we can calculate the number of moles of Neon needed:
n = PV/RT = (101.3 kPa * 3 L) / (0.0821 L·atm/mol·K * 273 K) = 3.7 moles
Therefore, 3.7 moles of Neon would be needed to maintain STP conditions in a 3 L container.
2. How many Cu atoms have a mass of 4.500x10^5 amu?
Answers
Answer:
63.55
Explanation:
4.500 x 10^5 amu x 1 Cu atom / 63.55 amu = 7081 Cu Atom
Cu 1 x 63.55 = 63.55
Which of the following accurately represents the relationship between ceramic and
metal?

Answers
Relationship between metal and ceramic is accurately represented by the statement that metal is a better conductor than ceramics.
What are conductors?Conduction is defined as a process as a means of which heat is transferred from the hotter end of the body to it's cooler end.Heat flows spontaneously from a body which is hot to a body which is cold. Substances which enable conduction are called conductors.Conductors allow passage heat and electricity through the material.
In the process of conduction,heat flow is within the body and through itself.In solids the conduction of heat is due to the vibrations and collisions of molecules while in liquids and gases it is due to the random motion of the molecules .
When conduction takes place, heat is usually transferred from one molecule to another as they are in direct contact with each other.There are 2 types of conduction:1) steady state conduction 2) transient conduction.According to the type of energy conduction is of three types:
1) heat conduction
2) electrical conduction
3)sound conduction
Learn more about conductors,here:
https://brainly.com/question/8426444
#SPJ6
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8