What tool would you use to test for hardness?
Answers
Answer: mohs picks
Explanation: hope its what you wanted
Related Questions
what is after gallium on the periodic table?
Answers
Answer:
Germanium or Ge
Explanation:
It is on the perioidic table. Gallium has an atomic number of 31 and Germanium has an atomic number of 32.
Name the molecular compound Si3N4
Answers
INFORMATION:
We have the next molecular compound
\(Si_3N_4\)And we must name it
STEP BY STEP EXPLANATION:
To name binary (two-element) molecular compounds, we must know the following information:
The first element in the formula is simply listed using the name of the element. The second element is named by taking the stem of the element name and adding the suffix -ide.
We can use the following table to specify the number of atoms in a molecule using a prefix
In our case,
the first element is Si: silicon and it has 3 atoms, so the first word of the name of the compound would be trisilicon.
the second element is N: nitrogen and it has 4 atoms, so the second word of the name of the compound using the suffix -ide would be tetranitride.
Finally, the name of the molecular compound is trisilicon tetranitride
ANSWER:
The name of the molecular compound Si3N4 is trisilicon tetranitride

Which of the following properties can only be measured in solids? PLEASE HELP ME
A.Shape and color
B.Size and color
C.Size and shape
D.Size and texture
Answers
Answer:
size and shape because solids nomally have a fixed shape and volume
I think it would be, C. Size and Shape.
Because, when measuring objects in solids u should measure the Size because the color is just paint and the texture is just how it feels. The shape can be measured in solids too.
So, your answer is C.
Good job if you made it this far!
Your reward?
I'll befriend you!
!
When writing the formula for an ionic compound, which element comes first?
Answers
Answer:
the cation comes first I believe
Explanation:Let me know if I'm wrong pls
4) Type only the NUMBER answer to the question below (no units). Your
answer should have 4 significant figures.
How many grams of N202 are present in 4.5 x 1024 molecules of N2O2
Answers
What is the valence e- (outermost e-) for the following elements:
a. Hydrogen
b. Carbon
c. Nitrogen
d. Neon
Answers
Answer:
A options hydrogen
Explanation:
valence outermost element is hydrogen.
Choose the pair of substances that are most likely to form a homogeneous solution.
A. F2 and C2H5OH
B. H2O and CH3OH
C. LiCl and C10H20
D. CH4 and C2H5OH
Answers
A) F2 and C2H5OH (Heterogeneous mixture)B) H2O and CH3OH (Homogeneous mixture)C) LiCl and C10H20 (Heterogeneous mixture)D) CH4 and C2H5OH (Heterogeneous mixture).
The pair of substances that are most likely to form a homogeneous solution is B. H2O and CH3OH. A homogeneous mixture is one in which the components are evenly distributed throughout the mixture. These types of solutions have uniform properties and the same composition throughout.A heterogeneous mixture is one in which the components are not evenly distributed. This means that the mixture has distinct regions or phases that are visibly different from one another. The following are the most likely pairs of substances to form a homogeneous solution:A) F2 and C2H5OH (Heterogeneous mixture)B) H2O and CH3OH (Homogeneous mixture)C) LiCl and C10H20 (Heterogeneous mixture)D) CH4 and C2H5OH (Heterogeneous mixture).
learn more about Heterogeneous
https://brainly.com/question/24898889
#SPJ11
What is the name of the ion with 4 protons and 2 electrons?.
Answers
The ion with 4 protons and 2 electrons is called a helium ion. A helium ion is an ion that contains two protons, two electrons, and no neutrons. The atomic number of helium is 2, indicating that it has two protons.
Helium is a colorless, odorless, tasteless, non-toxic, and inert gas that heads the noble gas series in the periodic table.The helium ion is an ion that has lost its two electrons and is, therefore, positively charged. The ionization of helium atom creates the helium ion. Helium ion is used in ion beam lithography, vacuum leak detection, and scanning helium microscopy because it has a relatively large atomic mass, a low reactivity, and a high mobility. In summary, the ion with 4 protons and 2 electrons is called a helium ion.
For more information on helium ion visit:
brainly.com/question/2886630
#SPJ11
In a blast furnace, iron(III) oxide reacts with coke (carbon) to produce molten iron and carbon monoxide: Fe2O3 + 3C → 2Fe + 3CO How many kilograms of iron would be formed from 132 kg of Fe2O3? kg
Answers
132 kg of Fe2O3 would produce 132 kg of iron in the reaction.
To determine the mass of iron formed from 132 kg of Fe2O3, we need to calculate the molar masses of Fe2O3 and Fe and then use stoichiometry to find the corresponding mass of iron.
The molar mass of Fe2O3 (iron(III) oxide) is:
2(Fe) + 3(O) = 2(55.85 g/mol) + 3(16.00 g/mol) = 159.69 g/mol
Now we can set up the stoichiometric ratio between Fe2O3 and Fe:
2 mol Fe2O3 : 2 mol Fe
The molar mass of Fe (iron) is:
Fe = 55.85 g/mol
Using the molar masses and the stoichiometric ratio, we can calculate the mass of iron formed:
132 kg Fe2O3 * (1,000 g / 1 kg) * (2 mol Fe / 2 mol Fe2O3) * (55.85 g / 1 mol Fe) = 132,000 g = 132 kg
Know more about stoichiometric ratio here;
https://brainly.com/question/6907332
#SPJ11
Multiply or divide to find the equivalent fraction.
51/120=□/360
Answers
Answer
153
Explanation:
51/120 = x/360
51(360) = 120x
18360 = 120x
18360/120x = 120x/120x
153 = x
Answer:
153
Explanation:
Give formulas corresponding to the following names: (a) Potassium tris(oxalato)chromate(III) (b) Tris(ethylenediamine) cobalt(III) pentacyanoiodomanganate(II) (c) Diamminediaquabromochloroaluminum nitrate
Answers
The formula of Potassium tris(oxalate)chromate(III) is K₃ [Cr (ox)₃] and Tris(ethylenediamine) cobalt(III)- [Co (en)₃]³⁺ [Mn (CN)₅]³⁻ and the formula of Diamminediaquabromochloroaluminum nitrate is [Al (NH₃)₂(H₂O)₂ Br Cl] NO₃⁻.
What are coordination compounds?Coordination compounds are formed by the coordination bond of metals with ligands which can be neutral or anionic. The coordination compounds have a coordination sphere in which the metal with bonded ligands are written in a square bracket.
They may have another ligand or metal outside the sphere which are written outside a square bracket. Here the first complex is Potassium tris(oxalate)chromate(III), where the K metal is outside the sphere and the ligand is oxalate which is a bidentate ligand with 2- charge. The formula of the compound is K₃ [Cr (ox)₃].
The second complex is Tris(ethylenediamine) cobalt(III) pentacyanoiodomanganate(II) which is an ionic pair of complexes. One anionic part and the cationic part and the formula is [Co (en)₃]³⁺ [Mn (CN)₅]³⁻ .
The last one is Diamminediaquabromochloroaluminum nitrate, where nitrate ion is outside the coordination sphere and the formula is [Al (NH₃)₂(H₂O)₂ Br Cl] NO₃⁻.
To find more on coordination complexes, refer here:
https://brainly.com/question/14240612
#SPJ1
Plssss helppp quick it’s for science and I don’t get it

Answers
High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to
purify chemical substances. The pressures used in this procedure range from around 500
kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know
the pressure in torr. If an HPLC procedure is running at a pressure of 3.20x10^8 Pa , what is its
running pressure in torr?
Express the pressure numerically in torr.
►
3.20 x 10^8 Pa = _____ torr

Answers
Answer:
2,400,000 torr (3 s.f.)
Explanation:
Convert the pressure from Pascal to atm first:
\(\boxed{1 \: atm = 101325 \:Pa }\)
3.20 ×10⁸ Pa
= [(3.20 ×10⁸) ÷101325] atm
= 3158.2 atm (5 s.f.)
Convert atm to torr:
\(\boxed{1 \: atm = 760 \: torr}\)
3158.2 atm
= (3158.2 ×760) torr
= 2400000 torr (3 s.f.)
Complete the sentence.
If it collects a lot of mass, a space object will become __________ due to __________.
Select one:
1. Spherical; electromagnetism
2. Spherical; gravity
3. A rocky planet; gravity
or
4. A rocky planet; electromagnetism
Answers
The answer to your question is 2
Balance the following chemical equation and explain how u got it
a. Fe + O2 →Fe2O3
b. FeBr3 + H2SO4 → Fe2(SO4)3 + HBr
c. C4H6O3 + H2O → C2H4O2
Answers
Explanation:
just trying...hope that helps

Sodium will react with chlorine to form sodium chloride (NaCl). Another cation, magnesium, will also react with chlorine to form magnesium chloride.

Answers
Answer:
See explanation below
Explanation:
Sodium will react with chlorine to form sodium chloride NaCl.
Na is a group I metal with 1 valance electron to become one Na+ ion to bond with one Cl- ion
Magnesium will also react with chlorine to form magnesium chloride MgCl2
Mg is a group II metal with 2 valence electrons become one Mg2+ ion to bond with two Cl- ions.
Both Na and Mg are metals while Cl is non-metals so the bond between metal and non-metal is ionic bond.
Hope this helps.
What is your carbon footprint?
the amount of carbon dioxide you use when recycling
the amount of oxygen put into the atmosphere due to your consumption of fossil fuels
the amount of carbon dioxide and oxygen in the atmosphere at any given time
the amount of carbon dioxide put into the atmosphere due to your consumption of fossil fuels
Answers
Answer:
c. .....................
Answer: the amount of carbon dioxide put into the atmosphere due to your consumption of fossil fuels
Your carbon footprint is the amount of carbon dioxide and other carbon compounds emitted, because of your use of fossil fuels.
Hope this helps!
Which of the following properties are most typical for nonmetals? Check all that apply.

Answers
Answer:
B) Poor conductors
Explanation:
According to the characteristics of non-metals , non-metals are poor conductors of electricity and are mostly gases at room temperature and are also brittle.
What are the characteristics of non-metals?
An element is the simplest form of matter.Elements are further classified as metal,non -metals and metalloids. Non-metals do not possess luster and are dull . They are not malleable and ductile and are soft due to the covalent bonding present in them . Non-metals owing to their soft nature have low melting points.
As they have large intermolecular spaces they are mostly gases at room temperature and are also brittle due to the presence of weak bonding present in them.They are poor conductors as they do not have mobile electrons.They have low tensile strength due to presence of covalent bonding.
Learn more about characteristics of non-metals,here:
https://brainly.com/question/16749127
#SPJ2
Define [Fluid compressibility, Solution-gas/liquid ratio, Fluid FVF, Fluid densities, and Fluid viscosities], write their equations, symbols, units \& correlations. (25-points)
Answers
1. Fluid compressibility (C): Fluid compressibility refers to the measure of how much a fluid's volume changes in response to a change in pressure.
2. Solution-gas/liquid ratio (SGLR): The solution-gas/liquid ratio represents the volume of gas dissolved in a given volume of liquid at a specific pressure and temperature.
3. Fluid formation volume factor (FVF): The fluid formation volume factor represents the ratio of the volume of a fluid at reservoir conditions (pressure and temperature) to its volume at surface conditions.
4. Fluid densities (ρ): Fluid densities refer to the mass per unit volume of a fluid.
5. Fluid viscosities (μ): Fluid viscosities represent the measure of a fluid's resistance to flow.
1. Equation: C = -1/V * dV/dP
Symbol: C
Unit: 1/Pascal (Pa^-1)
Correlation: The compressibility of fluids can vary depending on the fluid type. For ideal gases, the compressibility is inversely proportional to pressure.
2.Equation: SGLR = V_gas / V_liquid
Symbol: SGLR
Unit: Volumetric ratio (e.g., scf/bbl)
Correlation: The solution-gas/liquid ratio is influenced by the pressure and temperature conditions, as well as the composition of the fluid.
3. Equation: FVF = V_reservoir / V_surface
Symbol: FVF
Unit: Volumetric ratio (e.g., bbl/STB)
Correlation: The fluid formation volume factor depends on the composition and properties of the fluid, as well as the reservoir conditions.
4. Equation: ρ = m / V
Symbol: ρ
Unit: Mass per unit volume (e.g., kg/m^3)
Correlation: Fluid densities can vary depending on the type and composition of the fluid. For example, water has a density of approximately 1000 kg/m^3.
5. Equation: No single equation; viscosity is measured experimentally using viscometers.
Symbol: μ
Unit: Pascal-second (Pa·s) or centipoise (cP)
Correlation: The viscosity of a fluid is influenced by temperature and pressure. Different fluids exhibit different viscosities, ranging from low-viscosity fluids like water to high-viscosity fluids like heavy oil.
To know more about Fluid formation volume factor (FVF)
https://brainly.com/question/31458735
#SPJ11
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Which substances from the simulation are more alkaline than water?
Which

Answers
Answer:
The substances more alkaline than water are;
1) Spit, 2) Blood, 3) Hand Soap, and 4) Drain Cleaner
Explanation:
The diagram in the simulation displays a list of liquid substances, arranged from bottom to top in the order of increasing alkalinity.
The ph of water is 7.0
The ph of spit (saliva) is between 6.2 and 7.6
The ph of blood is about 7.4
The ph of hand soap is between 9 and 10
The ph of drain cleaner is between 12 and 14.
A The birthrate is higher than the death rate.
B The fertility rate is decreasing quickly.
C The immigration rate is higher than the emigration rate.
D The carrying capacity has been surpassed.

Answers
Answer:
A. The birthrate is higher than the death rate
Explanation:
name five organs of the digestive system and state there function
Answers
Answer:
Organ Movement Food Particles Broken Down
Stomach Upper muscle in stomach relaxes to let food enter, and lower muscle mixes food with digestive juice Proteins
Small intestine Peristalsis Starches, proteins, and carbohydrates
Pancreas None Carbohydrates, fats, and proteins
Liver None Fats
Explanation:
Which of the following statements is true?
A- Chemical digestion includes the physical breakdown of chunks of food.
B- Mechanical digestion begins in the mouth, and includes the tearing and grinding of food with teeth.
C- Mechanical digestion uses teeth to grind food and saliva to moisten the food
making it easier to chew.
D- Chemical digestion includes the chemical breakdown of food and mixing of the food particles with the help of the tongue
Answers
B- Mechanical digestion begins in the mouth, and includes the tearing and grinding of food with teeth.
C- Mechanical digestion uses teeth to grind food and saliva to moisten the food
making it easier to chew.
These points are true.
Assuming standard states for all reactants and products, determine thespontaneous direction of the following reactions by calculating the cell potential andfree energy:a. Cu + ZHCI = CUCI2 + H2b. Fe + ZHCI= FeCl2 + H2
Answers
a. The given reaction is spontaneous under standard conditions.
b. the reaction is spontaneous under standard conditions.
a. To determine the spontaneity of the reaction Cu + ZnCl2 -> CuCl2 + Zn, we need to calculate the cell potential and the Gibbs free energy change.
The half-reactions for this reaction are:
Cu -> Cu2+ + 2e- (E° = 0.34 V)
Zn2+ + 2e- -> Zn (E° = -0.76 V)
To obtain the overall cell potential, we subtract the reduction potential of the anode (Zn2+ + 2e- -> Zn) from the reduction potential of the cathode (Cu2+ + 2e- -> Cu):
E°cell = E°cathode - E°anode
E°cell = 0.34 V - (-0.76 V)
E°cell = 1.10 V
Since the cell potential is positive, the reaction is spontaneous under standard conditions.
To calculate the Gibbs free energy change, we use the equation:
ΔG° = -nFE°cell
where n is the number of electrons transferred in the reaction and F is the Faraday constant (96485 C/mol). For this reaction, n = 2.
ΔG° = -2 * 96485 C/mol * 1.10 V
ΔG° = -211.87 kJ/mol
Since the Gibbs free energy change is negative, the reaction is spontaneous under standard conditions.
b. To determine the spontaneity of the reaction Fe + ZnCl2 -> FeCl2 + Zn, we need to calculate the cell potential and the Gibbs free energy change.
The half-reactions for this reaction are:
Fe2+ + 2e- -> Fe (E° = -0.44 V)
Zn2+ + 2e- -> Zn (E° = -0.76 V)
To obtain the overall cell potential, we subtract the reduction potential of the anode (Zn2+ + 2e- -> Zn) from the reduction potential of the cathode (Fe2+ + 2e- -> Fe):
E°cell = E°cathode - E°anode
E°cell = (-0.44 V) - (-0.76 V)
E°cell = 0.32 V
Since the cell potential is positive, the reaction is spontaneous under standard conditions.
To calculate the Gibbs free energy change, we use the equation:
ΔG° = -nFE°cell
where n is the number of electrons transferred in the reaction and F is the Faraday constant (96485 C/mol). For this reaction, n = 2.
ΔG° = -2 * 96485 C/mol * 0.32 V
ΔG° = -62.02 kJ/mol
Since the Gibbs free energy change is negative, the reaction is spontaneous under standard conditions.
Learn more about spontaneous here :-
brainly.com/question/13790391
#SPJ11
3)What helps the plants to receive sunlight in tropical rainforests?
Answers
Answer:
Large leaves help plants to receive more sunlight when in tropical rainforests.
it may not be fair to compare the volume of an atom to the "b" parameter as there must be some "in-between" space when packing a mole of atoms as close as possible. this may make the volume of the "b" parameter appear a bit over ~10× greater than the volume of the atom. for instance, in the hexagonal close pack structure shown here, the volume taken up by a sphere of radius r is: vhcp
Answers
However, it is important to note that this comparison may not accurately reflect the actual volume difference between the atom and the "b" parameter.
When comparing the volume of an atom to the "b" parameter, it may not be fair to make a direct comparison. This is because when packing a mole of atoms as close as possible, there will be some "in-between" space.
This can make the volume of the "b" parameter appear greater than the volume of the atom.
In the hexagonal close pack structure, the volume taken up by a sphere of radius r can be calculated using the formula vhcp.
to know more about pack structure visit:
https://brainly.com/question/33223246
#SPJ11
The question is about the comparison of volume between an atom and the 'b' parameter.
Explanation:The subject of this question is Chemistry. It pertains to the comparison of the volume of an atom to the 'b' parameter. When packing a mole of atoms as close as possible, there is some 'in-between' space, which causes the volume of the 'b' parameter to appear greater than the volume of the atom.
An example of this is the hexagonal close pack structure, where the volume taken up by a sphere of radius r can be calculated using the formula vhcp.
Learn more about Volume comparison here:https://brainly.com/question/33844670
#SPJ12
Hey besties can y'all help me out
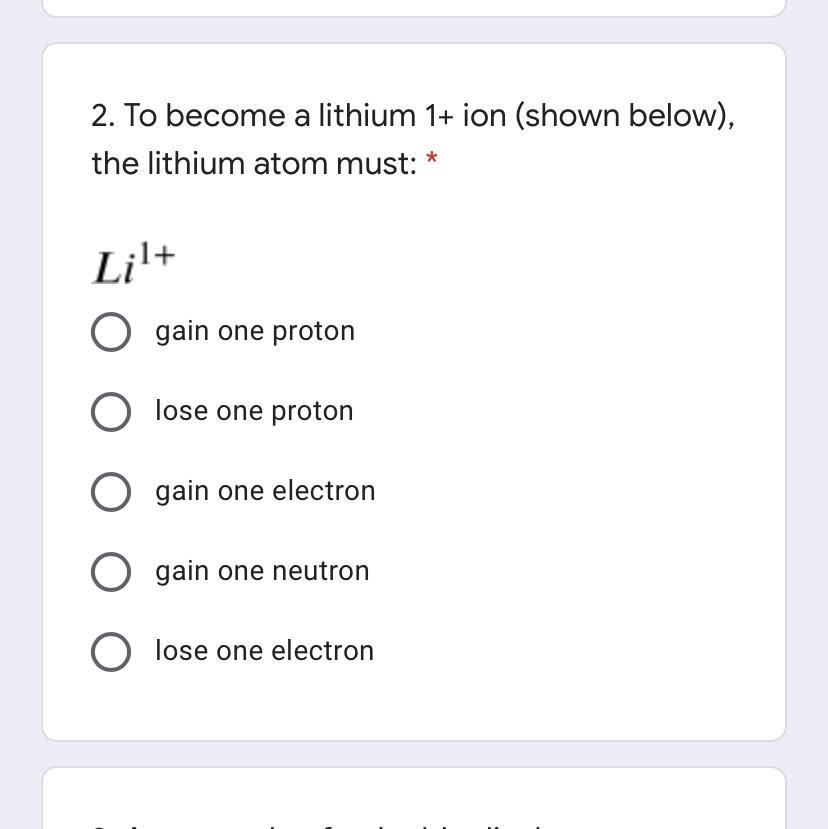
Answers
you need to lose one electron
Zinc reacts with 100.0 mL of 6.00 M cold, aqueous sulfuric acid through single replacement. How many grams of zinc sulfate can be produced?
Answers
The reaction between zinc and 100.0 mL of 6.00 M cold, aqueous sulfuric acid will produce 32.2 grams of zinc sulfate.
To determine the amount of zinc sulfate produced, we need to calculate the number of moles of zinc reacted and then use the stoichiometry of the balanced chemical equation to convert moles of zinc to moles of zinc sulfate. Finally, we can calculate the mass of zinc sulfate produced.
The balanced chemical equation between zinc and sulfuric acid is: Zn + H2SO4 -> ZnSO4 + H2. From the balanced equation, we can see that the molar ratio between zinc and zinc sulfate is 1:1.
First, calculate the number of moles of zinc reacted: moles of Zn = volume of H2SO4 × concentration of H2SO4
Since we have 100.0 mL of 6.00 M sulfuric acid: moles of Zn = 0.100 L * 6.00 mol/L = 0.600 mol
Since the molar ratio between zinc and zinc sulfate is 1:1, the number of moles of zinc sulfate produced is also 0.600 mol.
Finally, calculate the mass of zinc sulfate produced: mass of ZnSO4 = moles of ZnSO4 * molar mass of ZnSO4
The molar mass of zinc sulfate (ZnSO4) is: molar mass of ZnSO4 = (1 × atomic mass of Zn) + (1 × atomic mass of S) + (4 × atomic mass of O)
Using the atomic masses from the periodic table, the molar mass of ZnSO4 is approximately 161.4 g/mol: mass of ZnSO4 = 0.600 mol × 161.4 g/mol ≈ 96.8 grams
Therefore, approximately 32.2 grams of zinc sulfate can be produced from the reaction between zinc and 100.0 mL of 6.00 M cold, aqueous sulfuric acid.
To know more about reaction, refer here:
https://brainly.com/question/30464598#
#SPJ11
The tapir and agouti are ___________ found in the Amazon Rainforest.
A.Carnivores
B.Omnivores
C. Herbivores
D. Decomposers
"science"
Answers
C