What pair of elements have the same number of valence electrons?
a) Li and Na
b) Mg and K
c) Ti and Nb
d) Rb and Zr
Answers
The ans is A.
You need to refer back to the periodic table n find the 2 the is under the same grp.
Answer: its A
Explanation:
Related Questions
Spacing between atoms in a crystal is on the same order as the de Broglie wavelength of accelerated electrons.
A) observation a
B) observation b
C) observation c
D) observation d
E) observation e
The answer is D but I dont know why and I don't understand the question clearly
Answers
The de Broglie connection between both the momentum p or the wavelength of an electron (=h/p, h is Hubble constant) is used to calculate the wavelength of the an electron for a given power.
What is the accelerated electron formula?The following equation gives the kinetic energy of the an electron accelerated thru a voltage differential of V volts: where e is indeed the electron charge and 1/2 mv2 = eV (1.6x10-19 C) If you want to respond to this question, you'll need to know the electron charge as well as the Planck constant.
What is electron accelerated motion?Yet, a speeding electron creates a shifting magnetic field, which in turn causes a changing electromagnetic current, which in turn causes a shifting magnetic field, etc. Or to put it another way, it produces an electromagnetic wave.
To know more about wavelength visit:
https://brainly.com/question/31143857
#SPJ1
Calculate the frequency of wave that had a wavelength of 425 nm
Answers
The frequency of the wave is found to be 7.05×10¹⁴hertz.
The relation between speed of light, frequency and wavelength is given by,
C= fλ
f=c/λ
f= 3×10⁸/425×10⁻⁹
f=7.05×10¹⁴.
Thus, the frequency of the wave is found to be 7.05×10¹⁴ hertz.
The number of waves that pass a specific place in a given period of time is known as the wave frequency. The hertz (Hz) is the SI unit for wave frequency, and 1 hertz is equivalent to 1 wave crossing a fixed point in 1 second. A wave with a higher frequency has more energy than a wave with a lower frequency of the same amplitude.
A wave’s wavelength is the separation between two adjacent waves’ corresponding points. The Greek letter lambda () is typically used to represent a wave’s length. Wavelength is defined as the product of a wave train’s frequency (f) and speed (v) in a medium.
To learn more about wavelength, refer this link.
https://brainly.com/question/10750459
#SPJ9
Compare and contrast a solution and a suspension
Answers
The difference between a solution and a suspension is in the particle sizes involved. A solution is a mixture of ions or molecules (very, very small). Solutions are transparent, meaning that you can see through them. A suspension has bigger particle sizes and so it may look cloudy or murky
earth is one of the planets in the
Answers
Answer:
moon obviously
Explanation:
........
PLEASE ANSWER QUICK IT'S URGENT 40 POINTS!!!!!
What type of attractive force is the arrow pointing at in the molecule?

Answers
Answer: C intermolecular force
Explanation:
The type of attractive force shown in the figure is intermolecular force. Option C is correct.
There are nonbonding forces of attraction between one individual molecule and another. These forces are referred to as intermolecular forces and are responsible for the physical behavior of the phases of matter, such as their ability to form solids, liquids, and gases.
The strength of the intermolecular forces varies depending on the type of substance and its molecular structure. For example, substances with strong intermolecular forces, such as water, have a higher boiling point and are more likely to exist as liquids or solids at room temperature, while substances with weak intermolecular forces, such as methane, have a lower boiling point and are more likely to exist as gases.
To know more about intermolecular force here
https://brainly.com/question/32203220
#SPJ2
The half-life of cobalt-60 is 5.26 years. If a sample originally contained 220 grams of this isotope, how many grams would be left after 10.52 years?
Answers
Answer:
Question Answer
The half-life of cobalt-60 is 5.26 years. How many half-lives have passed in 10.52 years? 2
12.5% of a radioactive sample are left. How many half-lives have passed? 3
After 3 half-lives, how much of a 400 gram sample of radioactive uranium remains? 50g
Explanation:
What is the acceleration acquired by an object that has a mass of 50kg and is pushed with a force of 20N.
Answers
If a 20 N force is applied to a 50 kg mass, then the acceleration acquired by the body is 0.4 m/s².
¿How to calculate the acceleration of a body?It is possible to know the acceleration of a body from Newton's second law, which states that the acceleration is defined as:
a = F/mWhere:
A = accelerationF = forceM = massTroubleshooting:We proceed to find the acceleration of the body, such that:
a = F/ma = 20N / 50kga = 0.4m/s²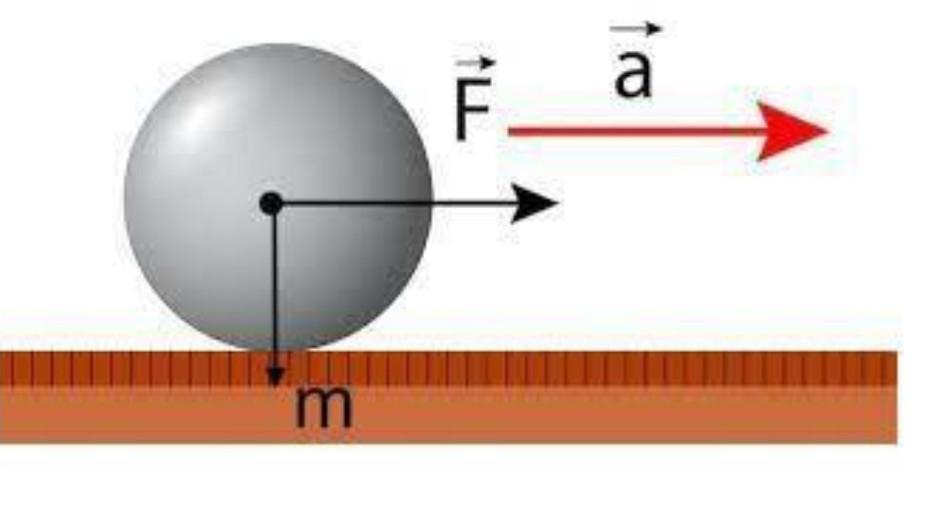
What does one mole of h20 correspond to
Answers
Answer:
One mole of H2O corresponds to 18 g .
In the solvolysis of 2-chloro-2-methylpropane, some di-t-butyl ether is formed. Explain this phenomenon in your own words and show the reaction sequence that represents this, starting with your starting materials.
Answers
In the solvolysis of 2-chloro-2-methylpropane, di-t-butyl ether formation occurs as a byproduct due to the interaction between the carbocation intermediate and a solvent molecule.
This is because the solvent used in the reaction, typically ethanol or water, can act as a nucleophile and attack the carbocation intermediate formed during the reaction. The carbocation intermediate is a positively charged species that is formed when the leaving group, in this case, the chloride ion, leaves the molecule.
When the nucleophile attacks the carbocation intermediate, it can form different products depending on the conditions of the reaction.
In the case of the solvolysis of 2-chloro-2-methylpropane, the nucleophile can attack the carbocation intermediate at either the carbon atom bearing the methyl group or the carbon atom bearing the tert-butyl groups.
If the nucleophile attacks the carbon atom bearing the methyl group, a molecule of ethanol or water is eliminated, resulting in the formation of di-t-butyl ether as a byproduct.
The reaction sequence for the solvolysis of 2-chloro-2-methylpropane can be represented as follows:
Starting material: 2-chloro-2-methylpropane
2-chloro-2-methylpropane + solvent (ethanol/water) → carbocation intermediate + leaving group (Cl-)
Carbocation intermediate + nucleophile (solvent) → di-t-butyl ether + solvent (ethanol/water)
As shown below;
Step 1: (C-Cl bond cleavage) → Tertiary carbocation + Cl⁻
Step 2: (Reaction with alcohol) → Di-t-butyl ether
Overall reaction:
2-chloro-2-methylpropane + solvent (ethanol/water) → di-t-butyl ether + leaving group (Cl-) + solvent (ethanol/water)
This side reaction competes with the main solvolysis reaction, leading to the formation of di-t-butyl ether in addition to the expected products.
To know more about solvolysis, click below.
https://brainly.com/question/22947698
#SPJ11
d. what happens when an iron nail is kept in copper sulphate solution?
Answers
Answer:
it changes color from blue to light green.
Explanation:
due to copper iron nail functions
Answer:
give the other person brainlieset plz
Explanation:
differentiate between Physical and chemical changes
Answers
Answer:
In a physical change the appearance or form of the matter changes but the kind of matter in the substance does not. However in a chemical change, the kind of matter changes and at least one new substance with new properties is formed.
I HOPE THIS WILL HELP YOU IF NOT THEN SORRY HAVE A GREAT DAY:)what happens to the rate if the concentration of chlorocyclopentane is tripled and the concentration of sodium hydroxide reamins the same
Answers
The rate of the reaction between chlorocyclopentane and sodium hydroxide will increase when the concentration of chlorocyclopentane is tripled and the concentration of sodium hydroxide remains the same.
This is due to the fact that increasing the concentration of a reactant increases the frequency of collisions between particles of the reactants, resulting in a higher reaction rate.
When a reactant's concentration is increased, the number of molecules or atoms per unit volume also increases. As a result, the frequency of collisions between the reactant particles increases.
The greater the frequency of collisions between the reactant particles, the greater the chance of a successful reaction, thus increasing the reaction rate.
When the concentration of one of the reactants is increased and the concentration of the other reactant remains the same, the reaction rate increases.
To know more about chlorocyclopentane click on below link:
https://brainly.com/question/14751180#
#SPJ11
20 POINTS!!
Kinetic and Potential Energy

Answers
Answer:
When a ball is rolled up a hill, it has potential energy that can turned into kinetic energy. The kinetic energy would be the ball rolling down the hill.
Explanation:
Hope this helps!
Calculate the maximum wavelength of light capable of dissociating the f–f bond in one molecule of fluorine if the bond energy, or bond dissociation energy, is 157 kj/mol.
Answers
The calculated maximum wavelength is 495 nm
The energy needed to break a bond and create two atomic or molecular fragments, each containing one of the original shared pair of electrons, is known as the bond dissociation energy. The link between silicon and fluorine, which was previously discussed, is discovered to be the strongest chemical bond. The initial silicon-fluorine bond in a silicon tetrafluoride molecule requires 166 kcal/mol of bond dissociation energy to be broken.
ΔH=+240 kJ/mol
E=242×103/6.022×1023
=4.0186×10−19 J
Now I use the Planck Expression:
E=hf=hc/λ
∴λ=hc
Eλ=6.63×10−34×3×108/4.0186×10-19 m
λ=4.949×10−7 m
λ=495 nm.
Learn more about dissociation energy here-
https://brainly.com/question/20475991
#SPJ4
By how much does the chemical potential of carbon dioxide at 310 K and 2.0 bar differ from its standard value at that temperature?
Answers
The standard value of the chemical potential of carbon dioxide at 310 K and 1 bar is considered to be zero. However, at 2.0 bar, the chemical potential of carbon dioxide will be slightly different due to the increased pressure. This can be calculated using the following equation:
Δμ = RTln(P/P°)
Where Δμ is the difference in chemical potential, R is the gas constant, T is the temperature in Kelvin, P is the actual pressure (2.0 bar in this case), and P° is the standard pressure (1 bar).
Substituting the values, we get:
Δμ = (8.314 J/mol*K) * ln(2.0/1) * (310 K)
Δμ = 590.4 J/mol
Therefore, the chemical potential of carbon dioxide at 310 K and 2.0 bar differs from its standard value at that temperature by 590.4 J/mol.
Learn more about chemical here:
https://brainly.com/question/30970962
#SPJ11
Question
Which atom would be neutral?(
Responses
an oxygen atom with 9 electrons, 8 protons, and 8 neutrons
an oxygen atom with 9 electrons, 8 protons, and 8 neutrons
an oxygen atom with 16 electrons, 18 protons, and 16 neutrons
an oxygen atom with 16 electrons, 18 protons, and 16 neutrons
an oxygen atom with 8 electrons, 8 protons, and 9 neutrons
an oxygen atom with 8 electrons, 8 protons, and 9 neutrons
an oxygen atom with 4 electrons, 6 protons, and 4 neutrons
Answers
Answer:
an oxygen atom with 8 electrons, 8 protons, and 9 neutrons
an oxygen atom with 8 electrons, 8 protons, and 9 neutrons
Explanation:
In order for atoms to be neutral, it has to have the same amount of electrons and protons
Kinetic energy is energy an object has because of its:
Question 1 options:
composition
position
density
motion
Answers
Answer:
I think its Motion
Explanation:
explain why the segment bc is signficantly shorter than segment de
Answers
Segments are an important concept in geometry, and they are defined as a portion of a line that has two endpoints. Without more information about the context in which these segments are located, we cannot definitively say why one is shorter than the other. However, we can use these examples to illustrate the importance of considering the endpoints and context when comparing segment lengths in geometry.
To start, we need to consider the endpoints of each segment. Segment BC has endpoints B and C, while segment DE has endpoints D and E. Next, we can look at the location of these endpoints in relation to each other.
One possibility is that segment DE is simply longer than segment BC because the distance between points D and E is greater than the distance between points B and C. However, this may not be the case since we are given no information about the relative positions of these points.
Another possibility is that the segments are located in different parts of a larger figure, and the surrounding geometry affects their lengths. For example, if segment DE is part of a triangle with a longer base than the triangle containing segment BC, then segment DE would be longer even if the endpoints D and E were closer together than the endpoints B and C.
Complete question - Explain why the segment bc is significantly shorter than segment de in a triangle?
For more such questions on Segments.
https://brainly.com/question/12961019#
#SPJ11
How many moles are there in 122 grams of NO2?
Answers
Answer:
1.47 × 10^(23) molecules
Explanation:
Which of the following are characteristics of a temperate broadleaf forest? (Select all that apply.)
They contain mainly coniferous trees.
They experience all four seasons.
They can be found along the Atlantic coast of the United States.
They are very dry and receive little to no rainfall.
Answers
Answer:
They can be found along the Atlantic coast of the United States.
They experience all four seasons.
Explanation:
The characteristic of temperate broadleaf forests are they can be found along the Atlantic coast of the United States and experience all four seasons.
What is temperate broadleaf forest?As the name suggested that temperate broadleaf forests are those forests in which temperature througout the year is moderate and leaf of the tress are broad in shape.
As in that forest temperature is mild but that forest will experience all four seasons.Quality of soil of this forest is rich in minerals.Tress which are mostly present in this forests are oak, maple, beech, hickory, etc which are having broad leaves.They are generally found in central China and eastern North America, means along the Atlantic coast of the United States.Hence they can be found along the Atlantic coast of the United States and experience all four seasons are the characteristics of this forest.
To know more about temperate broadleaf forests, visit the below link:
https://brainly.com/question/10008361
3. You just graduated high school. You've been offered a sweet job with a $30,000/yr salary.
Suddenly, another company offers you the same job for $18/hr. Assuming they have the same
benefits and are both full time, which is the better deal?
Conversion factors: full-time work is 49hr/wk, 52 wk/yr
Answers
Answer:
The second company job of $18 /hr is the better deal
Explanation:
We are told that;
-First job offers a salary of $30,000/yr
-Second job offers a salary of $18/hr.
Now, let's convert the salary of the second job to amount per year.
We are told that full-time work is 49hr/wk, 52 wk/yr.
Since we have 49 hrs in a week, then amount per week = 18 × 49 = $882 per week
Then amount per year = 882 × 52 = $45864 /yr
Thus, the second job pays $45864 /yr.
This is more than the first job. Thus, the second job is the better deal
Which of the three methods of recrystallization do you think will grow the largest crystals. Do you think the crystal growth by vapor diffusion or by layering will be better? Explain why.
Answers
The vapor diffusion method will grow the largest crystals
In comparing the three methods of recrystallization, which include slow evaporation, vapor diffusion, and layering, I believe that the vapor diffusion method will grow the largest crystals. This is because vapor diffusion provides a controlled, gradual change in concentration and temperature, allowing crystals to form and grow in a more organized manner. On the other hand, layering can also produce sizable crystals, but it might be less effective due to possible inconsistencies in the interface between the two solutions, which may affect the crystal growth. Overall, the vapor diffusion method offers the most favorable conditions for growing large, well-ordered crystals.
In summary, vapor diffusion is generally considered to be the method that promotes the growth of the largest crystals. The slow and controlled evaporation allows for a gradual supersaturation of the solute, resulting in the formation of larger and more well-defined crystals.
#SPJ11
An electric current transports 0.20 kC of charge in 14.0 minutes. Calculate the size of the electric current.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
Answers
What is the volume of 28.6g of a substance that has the density of 3.91g/cm cubed?
Answers
Answer:
The answer is
7.31 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \)
From the question
mass of substance = 28.6 g
density = 3.91 g/cm³
The volume is
\(volume = \frac{28.6}{3.91} \\ = 7.31457800\)
We have the final answer as
7.31 mLHope this helps you
Please help me out with this!!
I will mark you as brainliest!

Answers
suggest a reason why, when giving the rate of a chemical reaction, it is important to know that the reaction rate is an average reaction rate.
Answers
Understanding that the reaction rate is an average reaction rate allows for a more comprehensive analysis of the reaction kinetics, accounting for the complexities and variations that can occur throughout the reaction process.
It is important to know that the reaction rate is an average reaction rate because chemical reactions can occur at different rates at different stages of the reaction. The overall rate of a reaction is typically determined by the rate of the slowest step, known as the rate-determining step. However, other intermediate steps may occur at different rates.
Reaction mechanism: Complex chemical reactions often involve multiple intermediate steps. Each step may have its own rate, and the overall rate of the reaction is determined by the slowest step. By considering the average reaction rate, we can gain insights into the overall kinetics of the reaction and the rate-determining step.
Time-dependence: Reaction rates can change over time as reactants are consumed and products are formed. At the beginning of a reaction, the rate may be higher due to higher reactant concentrations. As the reaction progresses, reactant concentrations decrease, and the rate may slow down. By understanding the average reaction rate, we can account for these changes and obtain a more accurate representation of the reaction's kinetics.
Reaction conditions: Reaction rates can be influenced by various factors such as temperature, pressure, and the presence of catalysts. These factors can affect the rate of individual steps in the reaction mechanism. By considering the average reaction rate, we can take into account the impact of different conditions on the overall reaction rate.
Know more about reaction rate here:
https://brainly.com/question/13693578
#SPJ11
what is left over when energy is released from atp
Answers
When energy is released from ATP (adenosine triphosphate), the leftover molecule is ADP (adenosine diphosphate) and a free inorganic phosphate group (Pi).
Adenosine triphosphate (ATP), energy-carrying molecule found in the cells of all living things. ATP captures chemical energy obtained from the breakdown of food molecules and releases it to fuel other cellular processes.
1. ATP releases energy by breaking the bond between the second and third phosphate groups.
2. This reaction results in the formation of ADP (adenosine diphosphate) and a free inorganic phosphate group (Pi).
3. The released energy is used by the cell for various processes, while the ADP and Pi can be recycled to create more ATP when needed.
So, the leftovers when energy is released from ATP are ADP and Pi.
To learn more about ATP https://brainly.com/question/252380
#SPJ11
A total of 2. 00 mol of a compound i allowed to react with water in foam coffee cup and the reaction produce 173 g of olution
Answers
The enthalpy of this reaction is 1.10 kJ/mol.
What is enthalpy of reaction?The change in the enthalpy of a chemical reaction that takes place under constant pressure is known as the Heat of Reaction (also known as the Enthalpy of Reaction). It is a thermodynamic unit of measurement that may be used to determine how much energy is released or created per mole during a process. Enthalpy is a state function since it is generated from state functions, such as pressure, volume, and internal energy.By deducting the total enthalpies of all the reactants from the total enthalpies of the products, the reaction enthalpy is determined. According to mathematics, tH is equal to the sum of the enthalpies of the reactants and the product.Learn more about enthalpy of reaction refer to :
https://brainly.com/question/14047927
#SPJ4

5. which of the following statements concerning entropy is/are correct?1. the entropy of a substance increases when converted from a liquid to a solid.2. the entropy of a substance decreases as the number of atoms increases3. all substances have positive entropy values at temperatures above 0 k.
Answers
3. All substances have positive entropy values at temperatures above 0 K. this is correct statement.
Statement 1 is incorrect. The entropy of a substance generally increases when it is converted from a solid to a liquid. The increased freedom of movement and greater number of available microstates in the liquid phase lead to an increase in entropy.
Statement 2 is incorrect. The entropy of a substance generally increases as the number of atoms or particles increases. More atoms or particles contribute to a greater number of possible arrangements and microstates, resulting in higher entropy.
Statement 3 is correct. According to the third law of thermodynamics, all substances have positive entropy values at temperatures above absolute zero (0 K). At absolute zero, the entropy of a perfectly ordered crystal is considered to be zero, but as the temperature increases, the randomness and disorder (entropy) of the system also increase.
To know more about entropy visit:
brainly.com/question/20166134
#SPJ11
The mass of one methane molecule is 2.7×10⁻²³ gram. Find the mass of 50,000 molecules of methane. Express the answer in scientific notation. The mass of 50,000 molecules of methane is gram. (Use the multiplication symbol in the math palette as needed.)
Answers
The mass of 50,000 molecules of methane is 1.35 × 10⁻¹⁸ gram.
To find the mass of 50,000 molecules of methane, we need to multiply the mass of one methane molecule by the number of molecules.
Given:
Mass of one methane molecule = 2.7 × 10⁻²³ gram
Number of methane molecules = 50,000
To calculate the mass of 50,000 molecules of methane, we can use the following equation:
Mass = (Mass of one molecule) × (Number of molecules)
Mass = (2.7 × 10⁻²³ gram) × (50,000)
Now, let's calculate the mass:
Mass = 2.7 × 10⁻²³ × 50,000
Mass = 1.35 × 10⁻¹⁸ gram
Therefore, the mass of 50,000 molecules of methane is 1.35 × 10⁻¹⁸ gram.
Learn more about mass: https://brainly.com/question/21689106
#SPJ11