Answers
Water clarity is a measure of how far down light can penetrate through the water. The clearer the water is the farther light can go down. This is important because with water clarity we can find out how much living things that part of the water can support. Clearer water allows light to reach farther down, which can allow aquatic plants to grow deeper in the water.
Answer: Yes, if the water is not clear then it is most likely dirty
Related Questions
How to make crystals using table salt?
Please help I"ll mark you as the BRAINLIEST!!!
Answers
Answer:
1.heat a pan of water with just a little bit of water,have a boil
2.chosse ure salt
3.stir in has much salt has u can than take the pan off the heat
4.pour the mix into a glass jar
5.tie a string to an objeet that can lay accross the top and put just the string in ure mix
Explanation oh and look at it everyday hope that helps
Calculate the pH if [H+] = 4.51 x 10^-4 M
Answers
Explanation:
To perform calculations involving the pH of a solution, we can use the following logarithmic equation:
pH = - log [H3O+] or pH = - log [H+]
In calculations involving the pH of a solution, we always use logarithm to base 10.
In this case, we have:
[H+] = 4.51 x 10^-4 M
So:
pH = -log 4.51 x 10^-4
pH = 4 - log 4.51
pH = 4 - 0.65
pH = 3.35
Answer: pH = 3.35
What reaction does platinum catalyze in hydrogen fuel cells?
Answers
Answer:
Oxygen reduction reaction
Explanation:
Pt is used as the catalyst for both the hydrogen oxidation reaction (HOR) occurring at the anode and the oxygen reduction reaction (ORR) at the cathode. Usually, the Pt catalyst takes the form of small particles on the surface of somewhat larger carbon particles that act as a support.
All of the following changes to a metal are physical changes except_____.
bending
melting
rusting
polishing
Answers
Answer:
D)Polishing..
Answer:
Wouldn't it be rusting
Explanation:
Melting and bending are physical changes so is polishing. so rusting is the only choice, for metal to rust wouldn't it need a chemical change.
How many grams of methionine (MW = 149.21) are needed to make 20 mL of a 150 mM solution?
Answers
Answer:
0.45 g
Explanation:
Step 1: Given data
Molar mass of methionine (M): 149.21 g/molVolume of the solution (V): 20 mLConcentration of the solution (C): 150 mMStep 2: Calculate the moles of methionine (n)
We will use the following expression.
n = C × V
n = 150 × 10⁻³ mol/L × 20 × 10⁻³ L
n = 3.0 × 10⁻³ mol
Step 3: Calculate the mass of methionine (m)
We will use the following expression.
m = n × M
m = 3.0 × 10⁻³ mol × 149.21 g/mol
m = 0.45 g
How does a phase change affect a thermochemical equation?
O It alters the products.
O It alters the moles of reactants.
O It affects the balance of the equation.
O It can affect the AH value.
Answers
The correct answer is option D, It can affect the AH value.
What is a phase change?A phase change is a physical change in a substance in which the substance's state of matter is changed, such as from a gas to a liquid or from a liquid to a solid. It is also known as a phase transition.
Phase changes also involve changes in energy, temperature, and pressure. For example, when a solid melts to become a liquid, it absorbs energy and the temperature rises. When a liquid boils to become a gas, energy is released and the temperature decreases. Similarly, when a gas condenses to become a liquid, energy is released and the pressure increases.
Learn more about phase change here:
https://brainly.com/question/25664350
#SPJ1
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
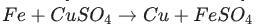
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
j.j. thompson experimented with alpha particles striking thin metal foils which led him to postulate the nuclear atom. True or False
Answers
False. J J. Thompson was not the one who experimented with alpha particles striking thin metal foils which led to postulate the nuclear atom.
It was actually Ernest Rutherford who conducted the famous gold foil experiment that led to the discovery of the nuclear atom. Rutherford and his colleagues bombarded a thin gold foil with alpha particles and observed that most of the particles passed straight through the foil, but a small number were deflected at large angles. This led Rutherford to conclude that the atom must have a small, dense, positively charged nucleus surrounded by mostly empty space.
J.J. Thomson, on the other hand, is credited with the discovery of the electron and the development of the "plum pudding" model of the atom, which was later replaced by Rutherford's nuclear model.
To know more about Ernest Rutherford refer here:
https://brainly.com/question/30666594#
#SPJ11
Relate the temperature of atmospheric gases to the production of rain.
Answers
Better temperatures can growth the quantity of water vapor withinside the air, that could growth the probability of precipitation.
Different factors, inclusive of air pressure, wind, and atmospheric instability, additionally play a function withinside the formation of rain, and the connection among temperature and precipitation may be complicated. The temperature of atmospheric gases could have a substantial effect at the manufacturing of rain. The environment is a complicated device that performs a vital function with inside the Earth`s water cycle, which incorporates the system of precipitation, inclusive of rain. Precipitation takes place while water vapor with inside the air condenses into liquid droplets or ice crystals, which fall to the floor as rain, snow, or hail. The temperature of the environment impacts the quantity of water vapor that the air can preserve. As temperature increases, the air can preserve extra water vapor, that could cause better tiers of humidity. When the air will become saturated with water vapor, it reaches its dew point, and the extra water vapor condenses into liquid droplets or ice crystals, that could shape clouds and subsequently precipitation. In addition, international warming, that's inflicting an growth in atmospheric temperatures, can cause modifications in precipitation styles and extra severe climate events. Understanding the connection among temperature and precipitation is vital for predicting and mitigating the affects of weather change.
For such more questions on precipitation
https://brainly.com/question/20469884
#SPJ11
I WILL GIVE 30 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The general gas equation, commonly referred to as the ideal gas law, represents the state of a fictitious ideal gas through an equation. Here the mass of helium gas required to pressurize 86 L tank to 201 atm is 2561.8 g.
According to the ideal gas law, the sum of the absolute temperature of the gas and the universal gas constant is equal to the product of the pressure and volume of one gram of an ideal gas.
The ideal gas equation is given as:
PV = nRT
n = PV / RT
56°C = 329 K
n = 201 × 86 / 0.08206 × 329 = 640.45 mol
Molar mass of 'He' = 4.00 g / mol
Mass = 640.45 × 4.00 = 2561.8 g
To know more about ideal gas law, visit;
https://brainly.com/question/30458409
#SPJ1
Please help.
Which organic product is formed in the reaction shown in the diagram?

Answers
The organic product formed in the reaction shown in the image would be bromoethane.
Substitution reaction in alkanesAlkanes, such as ethane undergoes a substitution reaction with halogens such as bromine to produce haloethanes.
For example, the equation of the reaction between ethane and bromine water is as below:
\(C_2H_6 + Br_2 --- > C_2H_5Br + HBr\)
The products formed are bromoethane and hydrobromic acid.
More on substitution reactions in alkanes can be found here: https://brainly.com/question/16811879
#SPJ1
Acetic acid and water react to form hydronium cation and acetate anion, like this: (aq)(l)(aq)(aq) Imagine of are added to a flask containing a mixture of , , and at equilibrium, and then answer the following questions. What is the rate of the forward reaction before any HCH3CO2 has been added to the flask
Answers
The question is incomplete. Here is the complete question.
Acetic acid and water react to form hydronium cation and acetate anion, like this:
\(HCH_{3}CO_{2}_{(aq)}+H_{2}O_{(l)} -> H_{3}O^{+}_{(aq)}+CH_{3}CO_{2}^{-}_{(aq)}\)
Imagine 226 mmol of \(CH_{3}CO_{2}^{-}\) are added to a flask containing a mixture of \(HCH_{3}CO_{2}\), \(H_{2}O\),\(H_{3}O^{+}\) and \(CH_{3}CO_{2}^{-}\) at equilibrium and then answer the following questions:
1) What's the rate of the reverse reaction before any \(CH_{3}CO_{2}^{-}\) has been added to the flask?
a) 0
b) Greater than 0, but less than the rate of the forward reaction
c) Greater than 0, but equal to the rate of the forward reaction
d) Greater than 0, but greater than the rate of the forward reaction
2) What is the rate of the reverse reaction just after the \(CH_{3}CO_{2}^{-}\) has been added to the flask?
a) 0
b) Greater than 0, but less than the rate of the forward reaction
c) Greater than 0, but equal to the rate of the forward reaction
d) Greater than 0, but greater than the rate of the forward reaction
3) What is the rate of the reverse reaction when the system has again reached equilibrium?
a) 0
b) Greater than 0, but less than the rate of the forward reaction
c) Greater than 0, but equal to the rate of the forward reaction
d) Greater than 0, but greater than the rate of the forward reaction
4) How much more \(CH_{3}CO_{2}^{-}\) is in the flask when the system has again reached equilibrium?
a) None
b) Some, but less than 226 mmol
c) 226 mmol
d) More than 226 mmol.
Answer: 1) c) Greater than 0, but equal to the rate of the forward reaction
2) d) Greater than 0, but greater than the rate of the forward reaction
3) c) Greater than 0, but equal to the rate of the forward reaction
4) b) Some, but less than 226 mmol
Explanation: A reversible chemical reaction reaches its equilibrium when forward and reverse reaction are at the same rate. At that point, equilibrium has a constant called K.
Equilibrium constant depends on the concentration of its products and reagents.
For the reaction \(HCH_{3}CO_{2}_{(aq)}+H_{2}O_{(l)} -> H_{3}O^{+}_{(aq)}+CH_{3}CO_{2}^{-}_{(aq)}\),
the forward is towards production of \(CH_{3}CO_{2}^{-}\) and reverse is towards the production of \(HCH_{3}CO_{2}\).
1) When equilibrium is reached, forward and reverse are at the same rate and are different from zero.
2) When adding a compound, the equilibrium is broken. So, to go back to the equilibrium, reaction tend to counteract the change. In the case of acetic acid and water above, when adding acetate anion, the reverse reaction will produce more acetic acid to restore equilibrium, so reverse reaction will be at a greater rate and different from 0.
3) After a while when the system is back to the equilibrium, the rate will be equal again.
4) After second equilibrium, acetate anion will have less mmol than when this new equilibrium state started.
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
if one gram of sulphur dioxide contains x molecules what will be the number of molecules in 1g of methane
Answers
The ratio of molecules in sulphur dioxide and methane will be the same as the ratio of their moles. So, first of all we should find out the number of moles of sulphur dioxide in 1 gram of sulphur dioxide in 1 gram of sulphur dioxide, and the number of moles of methane in 1 gram of methane. This can be done as follows :
(i) The molecular formula of sulphur dioxide is \(SO_{2}\)
So, \(1\) mole of \(SO_{2}\) = \(Mass\) \(of\) \(2'O'\)
\(=32+2*16\)
\(= 64\) grams
Now, \(64g\) of sulphur dioxide \(= 1\) mole
So, \(1g\) of sulphur dioxide = \(\frac{1}{64}\) mole
Thus, we have \(\frac{1}{64}\) mole of sulphur dioxide and it contains molecules in it. Now, since equal moles of all the substance contain equal number of molecules, therefore, \(\frac{1}{64}\) mole of methane will also contain x molecules of methane.
(ii) Molecular formula of methan is \(CH_{4}\)
So, 1 mole of \(CH_{4}\) = Mass of C + Mass of 4 H
\(=12+4*12\)
Now, 16g of methane = 1 mole
So, 1 g of mathane = \(\frac{1}{16}\) mole
We know that:
\(\frac{1}{64}\) mole of methane contains = x molecules
So, \(\frac{1}{16}\) mole of contains will contain =\(\frac{x*64}{16}\) molecules
=\(4x\) molecules
A weather balloon is filled with 28.6 L helium at sea
level where the pressure is 1.00 atm at 20.0 °C. The
balloon bursts after ascending until the pressure is 26.0
torr at -50.0 °C. Determine the volume (in L) at which
the balloon bursts.
Answers
The volume in which the weather ballon bursts is 640.21L.
How to calculate volume?The volume of a gas given the temperature and pressure can be calculated using the combined gas law as follows:
PaVa/Ta = PbVb/Tb
Where;
Pa, Va and Ta = initial pressure, volume and temperature respectivelyPb, Vb and Tb = final pressure, volume and temperature respectivelyAccording to this question, a weather balloon is filled with 28.6 L helium at sea level where the pressure is 1.00 atm at 20.0 °C.
1 × 28.6/293 = 0.034 × Vb/223
0.0976109215 × 223 = 0.034Vb
Vb = 21.767 ÷ 0.034
Vb = 640.21L
Learn more about volume at: https://brainly.com/question/24189159
#SPJ1
Consider the nuclear equation below.
Na-
22Ne +98
0
10
Which is the missing value that will balance the equation?
O-1
O 0
O +1
O +2

Answers
Answer:
+1
Explanation:
list at least 3 examples of giant Ionic structure and it's uses and application in today's industrial world.
Answers
The three examples of giant ionic structures used in industrial world today include. NaCl: sodium chloride. NaBr: sodium bromide. NaF: sodium fluoride.
What are giant ionic structures?A giant ionic structure is also known as an ionic compound which has a regular repeating arrangement called an ionic lattice.
Typical examples of giant ionic structures include the following:
NaCl (sodium chloride): It is used for manufacturing of household bleach, soaps, detergents and dyes in industries.NaBr (sodium bromide): Used as an antiseptic, detergent, and as reagent in pharmaceutical preparationsNaF (sodium fluoride): used to fluoridate water, in chemical cleaning and electroplating, and as an insecticide.Learn more about sodium here:
https://brainly.com/question/28106660
#SPJ1
How do you convert 134 kJ to Cal
Answers
That’s equal to 0,0320268 Cal.
Chose the element pairings that are likely to react with each other
Na and K or Na and Br
Answers
Answer:
Na k
Explanation:
because na is a metal and potassium is also a metal and both are active metal so is less likely to react as no bond is formed between them
Which molecule is more polar, RCH2COCH2R or RCH2(OH)R?
Answers
The answer would be RCH2COCH2R is more polar than RCH2(OH)R
The formic acid ( RCH2COCH2R ) is more polar than Pyridinium Chlorochromate (PCC) (RCH2(OH)R) because formic acid is from aldehyde group and is a reagent from a carbonyl group and PCC is react to form carbonyl group it's also an reagent which reacts in oxidation of alcohols.
The polarity is more which is a carbonyl compound as their electrons are loosely prepared. And also the polarity of formic acid is 6.0.
Hence, the formic acid (RCH2COCH2R) is more polar than Pyridinium Chlorochromate (RCH2(OH)R) (PCC).
To know more about Polarity here :
https://brainly.com/question/3184550?referrer=searchResults
#SPJ1
Of the four symbolic representations of different matter below, identify which best represents a single element.

Answers
Which of the following liquids has the weakest van der Waals forces of attraction between its molecules?
1
Kr(l)
2
Ne(l)
3
Ar(l)
4
He(l)
Submit Answer
Hide Toolbar
1
1
Zoom: Standard
Note
Bookmark
Eliminator
Highlighter
Calculator
Ruler
Line Reader
Reference
Answers
Answer:
He
Explanation:
This is because van der Waals force decrease down the group for noble gases. This is due to the ease of liquificaton down the group
H3C-CH2-C-CH2-CH2-CH3
CH-CH3
what is the formula of butane
Answers
Answer:
Butane : CH3 - CH2 - CH2 - CH3
Explanation:
The word Butane has "But" as preffix which stands for 4 carbon atoms & "ane" as suffix which tells that the compound is of alkane homologous series .

You have 400,000 atoms of a radioactive substance. After 3 half-lives have past, how many atoms remain? Remember that you cannot have a fraction of an atom, so round the answer to the nearest whole number.
Answers
Answer:
Explanation:
The number of atoms that remains after 3 half-lives given that it was originally 300000 atoms is 37500 atoms
Data obtained from the question
Original amount (N₀) = 300000 atoms
Number of half-lives (n) = 3
Amount remaining (N) =?
How to determine the amount remaining
The amount remaining after 3 half-lives can be obtained as illustrated below:
N = N₀ / 2ⁿ
N = 300000 / 2³
N = 300000 / 8
N = 37500 atoms
lewis structure of SeO3
Answers
Here it is, you know... Googling helps :)

The Lewis structure of SeO₃ is attached to the image below.
Selenium (Se) is in Group 16 of the periodic table, so it has 6 valence electrons. Oxygen (O) is in Group 16 as well, so each oxygen atom contributes 6 valence electrons. Since there are three oxygen atoms, the total number of valence electrons is:
6 (valence electrons of Se) + 6 (valence electrons of each O) 3 (number of O atoms)
= 6 + 18 = 24
The central atom in SeO3 is selenium (Se) since it is less electronegative than oxygen and can accommodate more than two bonded atoms.
Place the selenium atom in the center and connect it to each oxygen atom using a single bond. Each bond consists of two electrons.
Subtract the number of electrons used in step 3 from the total number of valence electrons: 24 (total valence electrons) - 6 (bonds) = 18
Distribute the remaining 18 electrons around the atoms to satisfy the octet rule.
To learn more about the Lewis structure, follow the link:
https://brainly.com/question/29603042
#SPJ6
Calculate the internal energy change, ΔrU, for the combustion of 29.3 g of vitamin C (C6H8O6, molar mass = 176.124 g mol-1) if the combustion inside a bomb calorimeter, Ccal = 8.31 kJ °C-1, causes a temperature change from 21.5 °C to 68.3 °C.
Answers
The internal energy change, ΔrU, for the combustion of 29.3 g of vitamin C (C6H8O6, molar mass is given as
-2.33810^3 kJ/mal
This is further explained below.
What is internal energy change,?Generally, The total of heat transmitted and work performed represents the change in the internal energy of a system. The change in the system's internal energy plus the PV work completed equals the heat flow.
Given
Mass of Vitamin C=29.3g
Molar mass of Vitamin C=176.124g/mol
C cal =8.31 KJJ^0
\(\Delta T &=68.3-21.5\)
=46.8^0
Bomb Calorimeter is a fixed volume, \(\Delta u=q+\omega=q\)
hence, heat generated by a reaction, denoted by released, equals heat generated by a calorimeter, denoted by again.
qreleased=-gained
\(=-C_{\text {cal }} \Delta T \\\)
Where
q= heat
T is temp
=-8.31* 468^o
=-388.908KJ
\($\begin{aligned} \text { No. of moles of Vitamin } C &=\frac{29.3 \mathrm{~g}}{176.124 \mathrm{~g} / \mathrm{mol}} \\ &=0.1663 \mathrm{moles} \end{aligned}$\)
No. of moles of Vitamin \(=\frac{29.3 \mathrm{~g}}{176.124 \mathrm{~g} / \mathrm{mol}}\)
=0.1663moles
On combustion of 29* 3 =0.1663 moles of Vitamin C,
\($\Delta u\)=-388.908KJs
Therefore Permole of Vitamin C,
\(\Delta u=\frac{-388.908}{0.1663}\)
=-2338.592
=-2.338 *10^3 KJ/mal
Read more about internal energy change,
at https://brainly.com/question/28024787
#SPJ1
this woman is riding a bicycle down a hill at a constant speed and in a straight line.
Which change will increase the speed of the bicycle?
A. Added forces of 30 N up the hill and 20 N down the hill
B. Added forces of 30 N up the hill and 30 N down the hill
C. An added force of 20 N down the hill
D. An added force of 20 N to the side of the hill

Answers
Answer:
what is your zoom email
teĺlllll
Calculate the molarity of a solution that contains 3.00x10-2 mol NH4Cl in exactly 450 cm3 of solution.
Answers
Answer:
0.06moles/litres
Explanation:
molarity = no of moles / volume in litres = 3.00*10^-2/(450/1000)=0.06 moles/litres
Answer:
0.07mol/dm^3
Explanation:
Please mark as brainliest

Review the equation below.
2KCIO3 > 2KCI + 3O2
How many moles of oxygen are produced when 2 mol of potassium chlorate (KCIO3) decompose?
A. 1
B. 2
C. 3
D. 6
The correct answer is C on edge 2021

Answers
Answer:
C) 3
Explanation:
Given data;
Number of moles of oxygen produced = ?
Moles of KClO₃ decomposed = 2 mol
Solution:
Chemical equation;
2KClO₃ → 2KCl + 3O₂
Now we will compare the moles of KClO₃ with O₂
KClO₃ : O₂
2 : 3
Thus, 3 moles of oxygen are produced.
How did max planck contribute to the modern atomic theory?
Answers
Answer:
Planck made many contributions to theoretical physics, but his fame rests primarily on his role as originator of the quantum theory. This theory revolutionized our understanding of atomic and subatomic processes, just as Albert Einstein's theory of relativity revolutionized our understanding of space and time
Answer:
revolutionized our understanding of atomic and subatomic processed