what is the ph of the solution when 0.2m hcl, 0.4m naoh and 0.2 m hcn is mixed, assuming the volume is constant. ( ka(hcn)= 5x10-10). give the answer in 3 sig. figs.
Answers
The pH of the solution is: pH = 14 - pOHpH = 14 - (-log0.2 - log V) = 14 + log0.2 + log V
The given data is as follows: 0.2M HCl, 0.4M NaOH and 0.2M HCN is mixed. Assuming the volume is constant, The Ka value of HCN is 5x10^-10.The pH of the solution when 0.2M HCl, 0.4M NaOH and 0.2M HCN is mixed can be determined as follows: First, let us write down the balanced chemical equation for the given reaction. HCl (aq) + NaOH (aq) → NaCl (aq) + H2O.
The equation above indicates that the amounts of HCl and NaOH are equal. Therefore, the number of moles of HCl present in the solution = 0.2 M × V (assuming 1 L volume). Similarly, the number of moles of NaOH present in the solution = 0.4 M × V. The amount of NaOH remaining after reacting with HCl is given by: Number of moles of NaOH remaining = 0.4 M × V – 0.2 M × V = 0.2 M × V The pH of the solution at this point is equal to the pOH, which can be calculated using the concentration of hydroxide ions in the solution.
To know more about solution visit:
https://brainly.com/question/15757469
#SPJ11
Related Questions
If the temperature of 68.8 g of ethanol increases from 40.0(C to 71.3(C, how much heat has been absorbed by the ethanol? (Specific heat of ethanol is 2.44 j/g((C)
Answers
Answer:
Q = 5254.39 J
Explanation:
Given that,
Mass of ethanol, m = 68.8 g
The temperature increases from 40.0°C to 71.3°C.
The specific heat of ethanol is 2.44 j/g°C
We need to find the heat absorbed by the ethanol. We know that the heat absorbed due to the change in temperature is given by :
\(Q=mc\Delta T\)
Put all the values,
\(Q=68.8\times 2.44\times (71.3-40)\\\\Q=5254.39\ J\)
So, 5254.39 J of heat is absorbed by Ethanol.
What was Leeuwenhoek talking about when he used the term " animalcules"?

Answers
Parents: Philips Antonisz van Leeuwenhoek, Margaretha Bel van den Berch
Died: 26 August 1723, Delft
Born: 24 October 1632, Delft
Nationality: Dutch Republic, Netherlands
Given the reaction: HSO4–(aq) + NH3(g) --> NH4+(aq) + SO42–(aq), which statement best describes the action of NH3(g) in the reaction?
Answers
Answer: NH3 (g) would be considered a base because it is a proton acceptor.
Explanation: at the end of the system, NH3 (g) turns into NH4+ (aq) which means it has accepted a hydrogen ion.
According to Bronsted- Lowry concept, bases are proton acceptors. Here, NH₃ is accepting a proton from HSO₄⁻. Hence, NH₃ is a base and option B is correct.
What is Bronsted- Lowry concept of acids and bases ?There are various concepts to define acids and bases. According to Bronsted - Lowry concept, acids are proton donors and bases are proton acceptors.
Acids forms their conjugate base by donating a proton and bases form their conjugate acid by accepting the proton from acid. Here, HSO₄⁻ acts as the acid to donate electron to ammonia.
Hence, NH₃ acts as the base to accept the proton from the acid and forms its conjugate acid NH₄⁺ (ammonium ion). Therefore, option B describes the action of NH₃.
Find more on Bronsted- Lowry concept:
https://brainly.com/question/12347903
#SPJ3
Your complete question is given below:

If you dive 66 ft underwater you will experience a pressure of 3.00 atmosphere. What is this in kilopascal?
Answers
If we are to express the pressure of the gas in kilopaschal then we have 303.9 kPa
What is pressure?We define the pressure of the gas as the force with which the gas is able to bump or hit or collide against the walls of the container in which the gas has been stored. In this case, we have been given the pressure of the gas but the unit in which the volume have been given is the unit of the atmosphere and we are asked by the question to express the pressure in the unit of kilopascal.
We know that from the use of the conversion factors;
1 atm = 101.3 kPa
3 atm = 3 atm * 101.3 kPa/ 1 atm
= 303.9 kPa
Learn ore about pressure of a gas:https://brainly.com/question/18124975
#SPJ1
what would be the freezing point of a solution containing 1.95 grams of biphenyl
Answers
The freezing point of a solution containing 1.95 grams of biphenyl would depend on the solvent used and the concentration of the solution.
The freezing point of a solution is lower than the freezing point of the pure solvent due to the presence of the solute. This is known as freezing point depression.
To calculate the freezing point of the solution, we would need to use the following formula:
ΔTf = Kf x molality
Where ΔTf is the change in freezing point, Kf is the freezing point depression constant for the solvent, and molality is the concentration of the solution in mol/kg.
First, we would need to calculate the molality of the solution by dividing the number of moles of biphenyl by the mass of the solvent in kilograms.
Molality = (1.95 g biphenyl / 154.21 g/mol) / (mass of solvent in kg)
Next, we would multiply the molality by the Kf constant for the solvent to find the change in freezing point.
ΔTf = Kf x molality
Finally, we would subtract the change in freezing point from the freezing point of the pure solvent to find the freezing point of the solution.
Freezing point of solution = freezing point of solvent - ΔTf
Without knowing the solvent and its freezing point depression constant, we cannot accurately calculate the freezing point of the solution containing 1.95 grams of biphenyl.
To know more about freezing points refer here:
https://brainly.com/question/3121416#
#SPJ11
what pertinent chemical information is needed in order to determine
if a chemical hazard truly exist?
Answers
In order to determine if a chemical hazard truly exists, there are several pertinent pieces of chemical information that must be considered. Firstly, the chemical composition of the substance in question must be examined, including its molecular formula and structural properties.
This information can help to determine the potential toxicity and reactivity of the chemical, as well as its potential routes of exposure (e.g. inhalation, ingestion, skin contact).
Additionally, data on the physical properties of the chemical - such as its melting point, boiling point, and solubility - can be important in determining how the substance may behave in different environments and under different conditions.
Finally, information on the potential environmental impact of the chemical, such as its persistence in the environment or its potential to bioaccumulate, can also be crucial in assessing the overall hazard posed by the substance.
By considering all of these factors together, a comprehensive picture of the potential hazards associated with a particular chemical can be developed, helping to inform appropriate risk management strategies and protective measures.
To know more about chemical hazard, refer here:
https://brainly.com/question/13340017#
#SPJ11
Elements on the periodic table are arranged in order by their
Answers
Answer:
in order of increasing atomic number, or the number of protons in an atom's nucleus, which generally coincides with increasing atomic mass
livesciencecom
Write a description (approx. 400 words) on any application of supercritical fluids (aside from the two noted below). It does not need to be a commercialised application. Include sketches (hand drawn or sketched yourself using some software, not copy-pasted) if needed. Submit on Moodle as a Word file or pdf. Neatly hand-written and then scanned/photo is also OK. Include references as appropriate, the format of the citations is not specified, but should be something reasonable (and consistent for all your citations). Note: it must NOT be one of the following two applications (i) Supercritical fluid extraction (e.g. scCO₂) of foodstuffs (e.g. caffeine) (ii) Supercritical water oxidation
Answers
Supercritical fluids have applications in nanoparticle synthesis, enabling precise control over size, shape, and composition.
One captivating use of supercritical liquids is in the area of nanotechnology, explicitly in the amalgamation and creation of nanoparticles. Supercritical liquids offer extraordinary benefits for nanoparticle development and handling because of their particular properties in the supercritical state.
Supercritical liquids can act as the two solvents and response media, taking into account the controlled blend of nanoparticles with positive size, shape, and sythesis. They can be utilized in different nanoparticle union strategies, like the supercritical liquid affidavit, supercritical antisolvent precipitation, and supercritical stage partition techniques.
In supercritical liquid testimony, nanoparticles are framed by infusing an answer containing forerunner materials into a supercritical liquid. The supercritical liquid goes about as a transporter medium and gives controlled conditions to the nucleation and development of nanoparticles.
The size and morphology of the nanoparticles can be custom-made by changing the working boundaries like temperature, strain, and stream rate.
Supercritical antisolvent precipitation includes dissolving the forerunner materials in a dissolvable and afterward quickly blending it in with a supercritical liquid that goes about as an antisolvent.
The abrupt decrease in dissolvability of the forerunners in the supercritical liquid outcomes in the precipitation of nanoparticles. This technique takes into consideration the blend of nanoparticles with exact command over their size, dispersity, and crystallinity.
Supercritical stage partition is one more strategy that uses the extraordinary properties of supercritical liquids. It includes the development of a biphasic framework by blending two immiscible parts, like a polymer and a dissolvable, in a supercritical liquid.
By changing the thermodynamic circumstances, nanoparticles can be framed at the point of interaction between the two stages. This technique offers a flexible methodology for the development of polymer-based nanoparticles with tunable properties.
The utilization of supercritical liquids in nanoparticle blend offers a few benefits. The supercritical state, first and foremost, gives amazing mass exchange properties, taking into consideration quick and uniform blending of reactants.
Furthermore, the shortfall of surface strain in supercritical liquids prompts the arrangement of nanoparticles with diminished agglomeration and further developed solidness. Also, supercritical liquids are harmless to the ecosystem, as they are non-poisonous and effectively recoverable.
All in all, the use of supercritical liquids in nanoparticle combination is a promising area of examination in nanotechnology. The one of a kind properties of supercritical liquids empower exact command over nanoparticle development, size, and structure.
This opens up opportunities for the improvement of cutting edge materials with custom-made properties for different applications like hardware, catalysis, drug conveyance, and that's only the tip of the iceberg.
To learn more about Supercritical fluids, refer:
https://brainly.com/question/31832043
#SPJ4
PLEASE HELP ME QUICKLY!
Imagine you are at your favorite beach. The sun is shining and you are enjoying the ocean breeze. The temperature is about 89F. You take your shoes off and realize that the sand is almost too hot to walk on. You run to the water's edge to wet your feet after the walk over the sand and realize that the water is almost too cold. Explain the temperature difference between the sand and water using Thermodynamics.
Answers
Answer:
The specific heat of water is more specific than the heat of sand, therefore, it will take more energy to raise the same amount of water with the same temperature.
Explanation:
The temp is 89 F so, 31.67° C
The specific heat of the water is 4.184 J/g° C
The specific heat of the sand is 0.290 J/ g° C
The heat is the amount of energy that is needed to raise the temp. of the substance
You will need a lot more energy to raise the temp of the water then of the same amount of sand, therefore, because of the specific lower heat of the sand it will raise it's temperature quicker compared to water.
What is true of a decomposition reaction? (4 points)

Answers
Answer:
C
Explanation:
It starts with one reactant to give and create new compounds as a product. They need a source of energy that can be heat, light, or electricity to break up chemical bonds.
Answer:
The addition of energy in the form of heat or electricity is often required.
Explanation:
A decomposition reaction is a chemical reaction that starts with single reactants to give new compounds as the products. These reaction requires a source of energy that could be light, heat or electricity to facilitate the breaking-down of the chemical bonds.
which acid or base is incorrectly identified as to type of compound? 1. ca(oh)2; weak base 2. hclo3; strong acid 3. hf; weak acid 4. h3po2; weak acid 5. csoh; strong base
Answers
The incorrect identification is number 1. Ca(OH)2 is actually a strong base, not a weak base. An explanation for this is that a strong base is one that completely dissociates in water, meaning that all of the molecules break apart into their constituent ions. Calcium hydroxide, Ca(OH)2, is one such compound that readily dissociates in water to produce calcium ions (Ca2+) and hydroxide ions (OH-). This makes it a strong base, rather than a weak base.
The compound that is incorrectly identified as to its type is:
1. Ca(OH)2; weak base
Calcium hydroxide, Ca(OH)2, is actually a strong base, not a weak base. The other compounds are correctly identified: HClO3 is a strong acid, HF is a weak acid, H3PO2 is a weak acid, and CsOH is a strong base.
To know more about chemical visit :-
https://brainly.com/question/11231920
#SPJ11
The acid or base that is incorrectly identified as to type of compound is ca(oh)2, which is labeled as a weak base.
Ca(oh)2 actually a strong base, not a weak base.
ca(oh)2 is incorrectly identified as a weak base.
Calcium hydroxide (Ca(OH)2) is actually a strong base, not a weak base as mentioned. The other compounds are correctly identified.
Hence, Ca(OH)2 was incorrectly identified as a weak base, but it is actually a strong base.
learn more about Calcium hydroxide click here:
https://brainly.com/question/619050
#SPJ11
Someone plz help I Don’t know I would do something like this and I really need to get it done
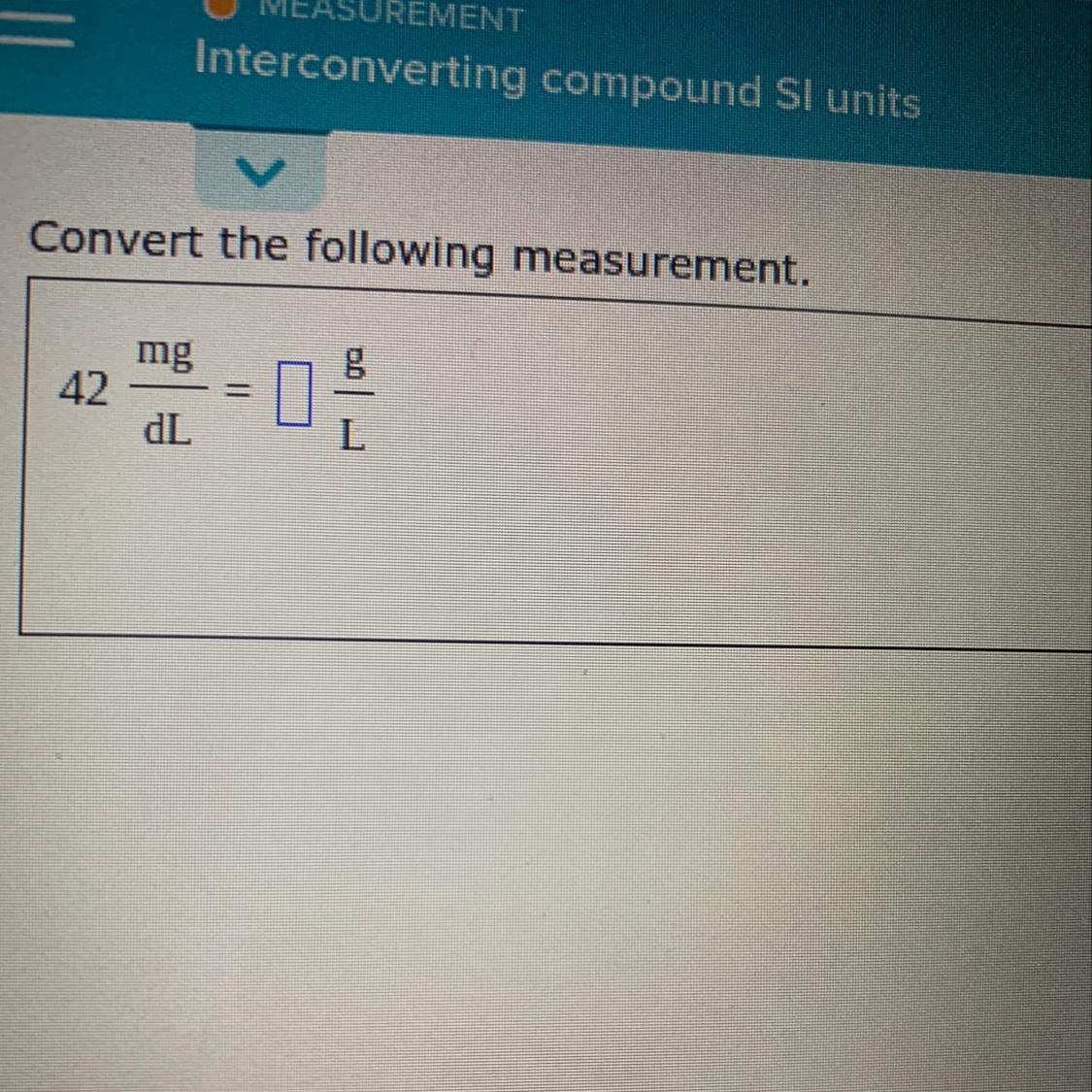
Answers
1dL =.1L
0.001/.1 *42=0.42
The hindbrain includes the:
A. limbic system.
B. brain stem.
C. corpus callosum.
D. occipital lobes.

Answers
The hindbrain consists of the brain stem which encompasses the medulla oblongata, pons, and cerebellum. The Option B.
What structures are included in the hindbrain?These structures are located at the base of the brain and are responsible for essential functions such as regulating vital autonomic processes, controlling balance and coordination .
It also relays sensory and motor information between the brain and the rest of the body. The hindbrain plays a crucial role in maintaining basic bodily functions and facilitating smooth movement. Therefore, the Option B is correct.
Read more about hindbrain
brainly.com/question/17584815
#SPJ6
Here are some last minute questions I need help with !

Answers
The balanced equations of the reaction are given below:
Overall equation: 2 H_O → H₂O₂ (l)
Equation 1: H_O ---> H_O (g)
Equation 2: H_O (g) → H₂O₂ (g)
Equation 3: H₂O₂ (g) → H₂O₂ (l)
What are balanced chemical equations?A balanced equation is an equation of a chemical reaction in which the number of moles of atoms for all the elements in the reaction is equal on both sides of the reaction.
A balanced chemical equation is obtained from the law of conservation of matter that states matter can not be created or destroyed.
Balancing chemical equations involves the addition of numerical coefficients to the reactants and products.
Learn more about balanced chemical equations at: https://brainly.com/question/11904811
#SPJ1
Calculate the ∆ S for the system when the state of 3 moles of an ideal gas for which Cpm=5/2 R is changed from 25°C and 1 atm to 125°C and 5 atm. How to rationalize the sign of ∆ S?
Answers
To calculate the change in entropy (∆S) for the system, we can use the formula: ∆S = nCp ln(T2/T1) + nR ln(V2/V1), ∆S = change in entropy of the system. n = number of moles of the gas.
Cp = molar heat capacity at constant pressure
R = gas constant (8.314 J/(mol·K))
T1, T2 = initial and final temperatures in Kelvin
V1, V2 = initial and final volumes
Given:
n = 3 moles
Cp = 5/2 R
T1 = 25°C = 298 K
T2 = 125°C = 398 K
P1 = 1 atm
P2 = 5 atm
First, we need to calculate the volume change (V2/V1). Since the problem does not provide any information about the volume change, we assume it to be constant. Therefore, V2/V1 = P1/P2
= 1/5
= 0.2.
Now, we can substitute the values into the entropy change formula:
∆S = (3 * (5/2) * R) * ln(398/298) + (3 * R) * ln(0.2)
Simplifying the equation:
∆S = (15/2)R * ln(398/298) + 3R * ln(0.2)
∆S = (15/2)R * ln(1.3356) + 3R * ln(0.2)
Using ln(1.3356) ≈ 0.29 and ln(0.2)
≈ -1.61:
∆S ≈ (15/2)R * 0.29 + 3R * (-1.61)
∆S ≈ 4.35R - 4.83R
∆S ≈ -0.48R
Therefore, the change in entropy (∆S) for the system is approximately -0.48 times the gas constant (R).
Rationalizing the sign of ∆S:
The negative sign indicates a decrease in entropy. In this case, the system has experienced a decrease in entropy as the gas has become more ordered. The gas has gone from lower temperature and pressure to higher temperature and pressure, suggesting that it has become more compressed and constrained. As a result, the gas molecules have less freedom of movement and fewer microstates available, leading to a decrease in entropy.
To learn more about entropy
https://brainly.com/question/419265
#SPJ11
Why is modern periodic table better than Mendeleev's periodic table?
Answers
Answer:
because mariah carey wrote it
Explanation:
she also made a song out of it!
True or False: Molar ratios come from the molar coefficients of balanced chemical reactions
True or False: The process of dimensional analysis requires the use of conversion factors *
True or False: The molar mass comes from balanced chemical reactions
Answers
Answer:
1. true
2.true
3.fasle
Explanation:
sorry if im wrong.
have a lovely day.
The Venn diagram compares organic and inorganic compounds.
Which statement belongs in the space marked X?
Made from phosphorus and oxygen
Not made from living things
Made from every element except carbon
Not made from elements

Answers
Answer:
i believe its the first one
Explanation:
Answer:
Not made from living things
Explanation:
(BRAINLIEST QUESTION)
Which answer below best describes what the law of Natural Selection is?
Select one:
a.
Natural selection is the process where those members of a species that can survive environmental hardships reproduce the future offspring with their strong characteristics being passed on, thus evolving the entire species.
b.
Natural selection is the process wherein species evolve over time because those members with common traits tend to survive hardships and produce more offspring.
c.
Natural Selection is the law that states the the natural evolution of a species is dependent on the shifts of the environment.
Answers
Answer:
I think it's A but I might be wrong
Explanation:
Write balanced chemical equations for dissociation of the following weak acids and identify their conjugate bases: phosphoric acid (H3PO4), formic acid (HCO2H), and boric acid (H3BO3).
Answers
The conjugate base of an acid is the negative ion that it forms in solution. The conjugate base of each of the following acids are indicated below;
1) H3PO4(aq) ⇄ H^+(aq) + H2PO4^-(aq)(conjugate base)
2) HCO2H(aq) ⇄ H^+(aq) + HCO2^-(aq) (conjugate base)
3) H3BO3(aq) ⇄ H^+(aq) + H2BO3^- (aq) (conjugate base)
What is a conjugate base?When an acid is dissolved in water it is dissociated into ions. The conugate base of the acid is the negative ion that it forms in solution. Let us now see the conjugate base of each of the ions formed;
1) H3PO4(aq) ⇄ H^+(aq) + H2PO4^-(aq)(conjugate base)
2) HCO2H(aq) ⇄ H^+(aq) + HCO2^-(aq) (conjugate base)
3) H3BO3(aq) ⇄ H^+(aq) + H2BO3^- (aq) (conjugate base)
Learn more about conjugate base: https://brainly.com/question/10468518
A 0.040 kg ball tied to a string moves in a circle that has a radius of 0.700 m. If the ball is accelerating at 43.2 m/s2, what is the tangential velocity of the ball?
Answers
Answer: 30.24 m/s
Explanation:
Got it right on test
What type of molecule is pentanal?
A. Aldehyde
O B. Ketone
O C. Alcohol
O D. Ester

Answers
Answer:
\( \huge \color{indigo} \boxed{a. \: aldehyde}\)
Explanation:
Pentanal is considered to be a fatty aldehyde lipid molecule. These are compounds containing more than one aldehyde group. Pentanal is a very hydrophobic molecule, practically insoluble in water, and relatively neutral.
how how lysine could be decarboxylated to give the end-products indicated. H2N COOH HH H H NH2 Lysine Cadaverine
Answers
Lysine can undergo decarboxylation to produce the end-product cadaverine.
Decarboxylation is a chemical reaction where a carboxyl group (-COOH) is removed from a molecule, resulting in the release of carbon dioxide (CO2). In the case of lysine, the decarboxylation reaction occurs at the carboxyl group (COOH) of the amino acid. The reaction can be catalyzed by enzymes known as decarboxylases. The chemical equation for the decarboxylation of lysine to cadaverine can be represented as follows:
H2N(CH2)4COOH (Lysine) → H2N(CH2)5NH2 (Cadaverine) + CO2
In this reaction, the carboxyl group (COOH) in lysine is removed, resulting in the formation of cadaverine, which has one less carbon atom and one less oxygen atom than lysine. It's important to note that decarboxylation reactions often require specific reaction conditions such as appropriate pH, temperature, and the presence of specific enzymes. Without these conditions, decarboxylation may not occur or proceed at a significant rate.
for more questions on Lysine
https://brainly.com/question/30666175
#SPJ8
which of the following statements correctly describe two enantiomers? (select all that apply.)
Answers
Enantiomers are non-superimposable mirror images of one another. Below are the statements that correctly describe two enantiomers: Enantiomers have the same chemical and physical properties except for their ability to rotate plane-polarized light in opposite directions.
Enantiomers are non-superimposable mirror images of one another. Below are the statements that correctly describe two enantiomers: Enantiomers have the same chemical and physical properties except for their ability to rotate plane-polarized light in opposite directions. The optical rotation of one enantiomer is positive and the other enantiomer is negative. Enantiomers have the same physical properties because they have the same functional groups and the same atomic connectivity.
The main difference between two enantiomers is the way in which they interact with other chiral molecules, such as proteins or enzymes, and this can lead to drastically different biological effects. For example, one enantiomer of a drug might be effective while the other enantiomer could be toxic. Therefore, it is important to separate enantiomers in the production of drugs to ensure safety and effectiveness.
In summary, enantiomers are mirror images of each other, but cannot be superimposed. They have the same physical properties and chemical formula, but different stereochemistry and interact differently with other chiral molecules, leading to different biological effects.
To know more about Enantiomers visit:
https://brainly.com/question/30401546
#SPJ11
When naming a compound The first element is?
Answers
While naming a compound, the less electronegative element is written first in the formula.
Generally, the less electronegative element is written first while writing the formula, though there are a few exceptions. Basically, carbon is always written first in a formula and hydrogen is after nitrogen in a formula such as NH₃. The common order of common non-metals in binary compound formulas is C, P, N, H, S, I, Br, Cl, O, F.
Generally, electronegativity is defined as a chemical property which describes the tendency of an atom or a functional group to attract electrons toward itself. The electronegativity of an atom is basically affected by both its atomic number and the distance that its valence electrons reside from the charged nuclei. And the more electronegative element is written in the first name of the compound.
Learn more about electronegativity from the link given below.
https://brainly.com/question/17762711
#SPJ4
Please help me with this.
Question 4 of 10 Which procedure would best demonstrate that a moving magnet causes an electric current to flow in a wire coil? O A. Connect a wire coil to an ammeter. Move a bar magnet into and out of the wire coil as you observe the ammeter. B. Connect a wire to a lightbulb, a battery, and a magnet on a rotating turntable. Turn on the switch as you observe the lightbulb. C. Connect a battery to a wire and an ammeter. Move a bar magnet in circles around the battery as you observe the ammeter. D. Connect a wire to a lightbulb and a bar magnet. Turn on the switch as you observe the lightbulb.
Answers
Answer A: Connect a wire coil to an ammeter. Move a bar magnet into and out of the wire coil as you observe the ammeter.
1: A50 L balloon is at 4 atm of pressure. What is the volume at 2 atm of pressure?
Answers
Answer:
100 L
Explanation:
Use Boyle's Law, p1v1 = p2v2.
p1 = 4 atm
v1 = 50L
p2 = 2 atm
v2 = ?
p1v1 = p2v2
(4 atm) (50L) = (2 atm) (v2)
v2 = (4 atm)(50L) / (2 atm) = 100 L
what is the heat in joules required to melt 25 grams of ice?
Answers
Answer:
8,350 Joules
Hope this helps
what elements on the periodic table have atomic numbers that add up to 200.
100 points
Answers
The elements on the periodic table that have atomic numbers adding up to 200 are gold (Au) and thulium (Tm).
1. Start by examining the periodic table, which lists all known elements in order of increasing atomic number.
2. Look for elements with atomic numbers that add up to 200.
3. Begin with the element at the lowest atomic number and work your way up.
4. Scan the periodic table until you find an element with an atomic number that is close to but less than 200.
5. Identify the element with the atomic number closest to 200, ensuring that the sum of the two atomic numbers does not exceed 200.
6. After scanning the periodic table, you will find that gold (Au) has an atomic number of 79.
7. Now, search for another element with an atomic number that, when added to the atomic number of gold, equals 200.
8. Continuing the search, you will discover that thulium (Tm) has an atomic number of 69.
9. Add the atomic numbers of gold (79) and thulium (69) together: 79 + 69 = 148.
10. Confirm that the sum of the atomic numbers equals 200 (148 + 52 = 200), satisfying the given condition.
11. Therefore, the elements on the periodic table that have atomic numbers adding up to 200 are gold (Au) and thulium (Tm).\
For more such questions on atomic, click on:
https://brainly.com/question/30390726
#SPJ8
The elements on the periodic table that have atomic numbers adding up to 200 are Polonium (Po), Gold (Au), and Thallium (Tl).
Explanation:The elements on the periodic table that have atomic numbers that add up to 200 are:
Polonium (Po): atomic number 84Gold (Au): atomic number 79Thallium (Tl): atomic number 81The sum of the atomic numbers of these elements is 84 + 79 + 81 = 244, not 200.
Learn more about atomic numbers on the periodic table here:https://brainly.com/question/35902425
A car travels 180 m souteast in 30 s. What is it's average velocity?
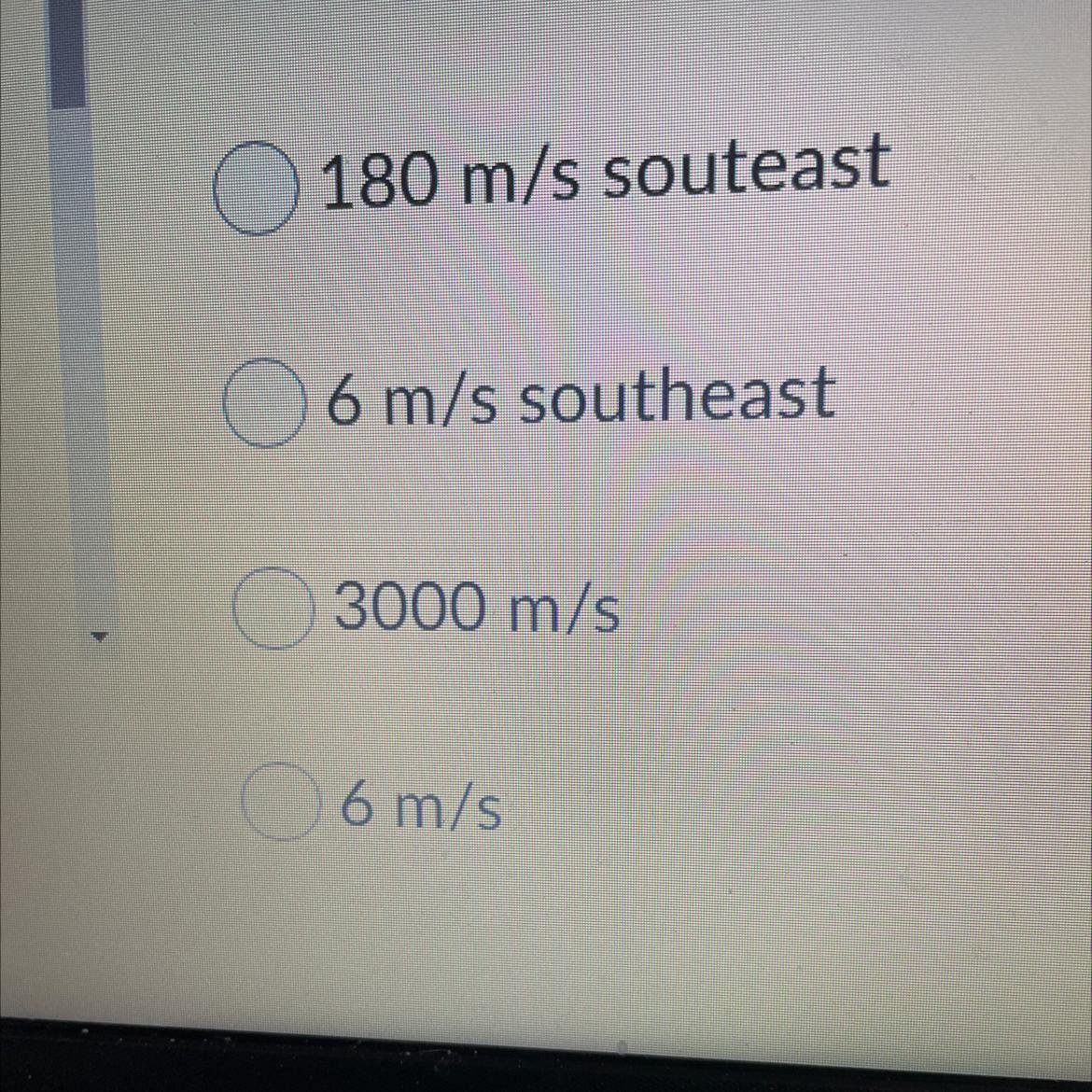
Answers
Answer:
B. 6m/s southeast
Explanation:
velocity= distance / time
= 180/ 30
6m/s southeast