What is the percent by mass of oxygen (O) in NaHCO3?
Answers
Answer: 57.13%
Explanation:
Related Questions
Pls answer I am giving lots of points and need all the work shown. Determine the kb for chloroform when 0.793 moles of solute in 0.758 kg changes the boiling point by 3.80 °C.
Answers
Answer:0.87
Explanation:
Jenna took an open bowl of leftover mashed potatoes from the refrigerator and noticed a difference in smell. She determined that chemical changes occurred since the potatoes were first placed there.
Which observations most likely led to Jenna’s conclusion?
Answers
Answer:
The change in smell
Explanation:
chemical reactions ccan lead to change in temperature, change in color and also change in smell
What is the molecular formula of the compound CH2 with molar mass = 42.0 g/mol? A. C2H18 B. C2H4 C. CAHg O D. C3H6
Answers
The molecular formula of the compound CH2 with molar mass = 42.0 g/mol is C₃H₆. Therefore, option D is correct.
What is molar mass ?The term molar mass is defined as the mass in grams of one mole of a substance. The units of molar mass are grams per mole, abbreviated as g/mol.
First, let's find the molar mass of CH₂.
The molar mass CH₂= 12.0 + (2 x 1.01)
= 14.02 g/mol
Now to determine the molecular formula, we need to find the ratio based in the molar masses.
42 / 14.02
= 2.99
≅ 3
Therefore, the ratio is 3, the molecular formula is C₃H₆.
Thus, option D is correct.
To learn more about the molar mass, follow the link;
https://brainly.com/question/12127540
#SPJ1
What does Hess's law state? A. The enthalpy of a reaction is positive if it is endothermic and negative if exothermic. B. The enthalpy of a reaction is the sum of the enthalpies of intermediate reactions. C. The enthalpy of a reaction can be determined only from single- step reactions. D. The enthalpy of a reaction can be measured from heat produced in a calorimeter.
Answers
Hess's law gave the net change in enthalpy. According to the law, the enthalpy of the reaction is the addition of the enthalpies of the intermediate reaction. Thus, option B is accurate.
What is Hess's law?Hess's law or the law of constant heat summation is the measure of the heat or the enthalpy of the chemical reaction. It was given by the chemist, Germain Hess.
The law states that the enthalpy of the chemical reaction is the sum of the enthalpies involved in the reactants and the products of the reaction. It is independent of the steps involved in the reaction.
Therefore, option B. enthalpy of reaction is the summation of the enthalpy of the intermediates.
Learn more about Hess's law here:
https://brainly.com/question/19480954
#SPJ2
When electrons are removed from the outermost shell of a calcium atom, the atom becomes
A)an anion that has a larger radius than the atom.
B) an anion that has a smaller radius than the atom.
C)a cation that has a larger radius than the atom.
D) a cation that has a smaller radius than the atom.
Answers
Answer:
A cation that has a smaller radius than the atom.
Explanation: when electrons are removed from the outermost shell of a calcium atom, the atom becomes a cation that has a smaller radius than the atom.
When electrons are removed from the outermost shell of a calcium atom, the atom becomes a cation that has a smaller radius than the atom. Therefore, the correct option is option D.
What is atom?Atom, the smallest unit of matter that may be separated without releasing electrically charged particles. It is also the smallest unit of matter with the characteristics of a chemical element. As a result, the atom is indeed the fundamental building component of chemistry.
The majority of the atom comprises empty space. The rest of the structure is made up of a positive-charged nucleus of neutrons and protons surrounding by a cloud of electrons with negative charges. In comparison to electrons, the lightest energized particles in nature, the nuclei is small and dense. When electrons are removed from the outermost shell of a calcium atom, the atom becomes a cation that has a smaller radius than the atom.
Therefore, the correct option is option D.
To know more about atom, here:
https://brainly.com/question/29712157
#SPJ6
Classify each of the following diatomic species as ionic, polar covalent or nonpolar covalent. Bra CsBr NaCl Cao HCI 02 KE HBr Ng BIF lonic Polar Covalent Nonpolar Covalent Categorize each of the following molecules according to what type of exception to the octet rule it is. SO, XeF. BeCl3 NO, NO OCI PFS NH, AICI BF, PO CF Electron Deficient Odd Electron Species Expanded Octet Obeys the octet rule
Answers
The Nernst equation connects the standard cell potential to the effective concentrations (activities) of the reaction's constituent parts. Br2 is a nonpolar covalent substance. Ionic substances include CsBr, NaCl, CaO, HCl, O2, KF, and HBr. Polar substances include HBr.
What do you mean by molecule?With this designation, the phrase's original definition—"the smallest unit of a material that yet preserves the attributes of that substance"—would be fully encapsulated. An atom is a body that cannot be divided into two, and a molecule is the tiniest unit of a certain material, according to James Maxwell's definition from 1873. A molecule is a collection of two or more atoms that constitutes the smallest discernible unit into which a pure substance can be divided while retaining its chemical makeup and other physical characteristics. molecular structure illustrations.
How many is a molecule?A group of two or more atoms bound together by the attractive forces known as chemical bonds is referred to as a molecule; depending on the context, the term may or may not include ions that meet this requirement. When two or more atoms of the same or different element are joined together, they form a molecule. A molecule can be homonuclear, meaning it is made up of atoms of the same chemical element, such as oxygen (O2), or it can be heteronuclear, meaning it is made up of more than one element, such as water (H2O).
To know more about Molecule visit:
https://brainly.com/question/19922822
#SPJ4
what particles are isotopes of each other
Answers
Isotopes are atoms of the same element with the same number of protons and electrons but varying atomic weights and neutron counts. The isotopes of an element have the same chemical characteristics because they share the same number of electrons, but because of their various atomic weights, they have varied physical characteristics.
Atoms with the same atomic number but varying atomic masses are known as isotopes. They are substances that differ in the quantity of neutrons they contain despite having the same amount of protons and electrons. The isotopes of an element have the same chemical characteristics because they share the same number of electrons, but because of their various atomic weights, they have varied physical characteristics. For instance, the carbon isotopes C-12, C-13, and C-14 contain varying amounts of neutrons but the same number of protons and electrons (six each). There are 6 neutrons in C-12, 7 in C-13, and 8 in C-14. Another illustration is the isotopes of oxygen, which are O-16, O-17, and O-18. While they each have 8 protons and 8 electrons, they have different numbers of neutrons. O-16 contains eight neutrons, O-17 nine, and O-18 ten. The identical amount of electrons among these isotopes determines the element's chemical characteristics. Isotopes are helpful in a variety of industries, including nuclear power, medical imaging, and radiocarbon dating. For instance, radiocarbon dating uses carbon-14 to establish the age of ancient artefacts, while iodine-131 is used in medical imaging to diagnose and treat thyroid problems.For more such questions on Isotopes , click on:
https://brainly.com/question/14220416
#SPJ8
NADPH inhibits the ____ pathway
Answers
NADPH inhibits the pentose phosphate pathway
NADPH is a product of the pentose phosphate pathway, which generates NADPH and ribose-5-phosphate.
NADPH is a coenzyme that plays a vital role in many metabolic processes, including the pentose phosphate pathway.
Therefore, it is incorrect to say that NADPH inhibits the pentose phosphate pathway. Instead, NADPH is a key participant in this pathway, where it is produced and utilized to synthesize nucleotides, amino acids, and fatty acids, and to protect cells against oxidative stress
When there is an excess of NADPH, it can inhibit the pentose phosphate pathway through feedback inhibition, thus reducing the production of NADPH. This is important to maintain a balance of NADPH and other metabolites in the cell.
To know more about pentose phosphate visit:
brainly.com/question/30500750
#SPJ11
2. Predict: Set the number of Oz molecules to five and the number of H2 molecules to eight.
A. How many oxygen atoms are present?
Hydrogen atoms?
B. How many water molecules could form from these reactants?
C. After the molecules react, which reactant will be left over?
D. Which reactant will be the limiting reactant?
E. Click Play and wait until Reaction complete is shown. What happened?
Answers
A. If there are five O₂ molecules, each O₂ molecule consists of two oxygen atoms. Therefore, the total number of oxygen atoms would be 5 × 2 = 10.
If there are eight H₂ molecules, each H₂ molecule consists of two hydrogen atoms. Therefore, the total number of hydrogen atoms would be 8 × 2 = 16.
B. For water (H₂O) to form, it requires two hydrogen atoms and one oxygen atom. Since there are only 10 oxygen atoms available, the maximum number of water molecules that can form is limited by the number of oxygen atoms. Therefore, the maximum number of water molecules that can form is 10/1 = 10.
C. After the reaction, if all the available oxygen atoms react with hydrogen atoms to form water molecules, there will be no oxygen or hydrogen left over.
D. Since there are 10 oxygen atoms available and only 8 hydrogen atoms, the hydrogen is the limiting reactant. This means that the reaction will stop once all the hydrogen is consumed, and there will be some excess oxygen atoms remaining.
E. As an AI text-based model, I'm unable to perform real-time simulations or reactions. Therefore, I cannot provide information on what happens after the reaction completes.
Learn more about H₂ here:
https://brainly.com/question/32509855
#SPJ11
At STP, what volume of carbon dioxide is produced from the complete combustion of 5.42 moles of ethanol?
C2H6O + 3 O2 2 CO2 + 3 H2O
Group of answer choices
A.121 L
B.365 L
C.243 L
D.60.7 L
Answers
Answer:
A
Explanation:
1mole=22.4L
(22.4/1mole)×5.24
=117,376L
If I have 8.3 moles of gas at a pressure of 9 atm and at a temperature of 62°C, what is the volume of the container that the gas is in?
Answers
Explanation:
To calculate the volume of the container that the gas is in, we can use the ideal gas law, which is given by the equation:
PV = nRT
where:
P = pressure of the gas (in atm)
V = volume of the gas (in liters)
n = amount of gas in moles
R = ideal gas constant (0.0821 L·atm/(mol·K))
T = temperature of the gas (in Kelvin)
First, we need to convert the given temperature from Celsius to Kelvin by adding 273.15 to it:
T = 62°C + 273.15 = 335.15 K
Now we can plug in the given values into the ideal gas law equation and solve for V:
P = 9 atm
n = 8.3 moles
R = 0.0821 L·atm/(mol·K)
T = 335.15 K
PV = nRT
9 V = 8.3 * 0.0821 * 335.15
V = (8.3 * 0.0821 * 335.15) / 9
V ≈ 26.79 liters
So, the volume of the container that the gas is in is approximately 26.79 liters.
I need help with this question. Thank you!

Answers
Answer:
J. All of these
Explanation:
I hope it helps.
I think it would be "all of the above" because all the things the wild hogs do are impacting all of the answers stated there.
hope that helped!!
What is the freezing point, in °C, of a solution made with 1.31 mol of CHCl₃ in 530.0 g of CCl₄?
Answers
Answer:
-96.6°C
Explanation:
Given:
moles CHCl3 = 1.31 molmass CCl4 = 530.0 gmass CCl4 = 530.0 g * (1 kg / 1000)
mass CCl4 = 0.5300 kg
First calculate the molality (m) of the solute, which is the moles of solute divided by the kg of solvent (\(\frac{moles}{kg}\)):
molality CHCl3 = (mass moles CHCl3) / (mass CCl4 in kg)
molality CHCl3 = (1.31 mol) / (0.5300 kg)
molality CHCl3 = 2.471698m
Decrease in freezing point of solvent = (Kf) * (molality of solute)
Decrease in freezing point of CCl4 = (Kf) * (molality CHCl3)
Decrease in freezing point of CCl4 = (29.8°C/m) * (2.47m)
Decrease in freezing point of CCl4 = 73.6566°C
Then use the molality of the solute to calculate the change in freezing point and subtract the change from the original freezing point.
Freezing point of solution =
(freezing point of pure solvent) - (Decrease in freezing point)
Freezing point of solution = (-22.9°C) - (73.66°C)
Freezing point of solution = -96.6°C
Learn more about freezing point here: brainly.com/question/22888016


Which one is correct...
1: Food mass is stored as mass, and then destroyed
2: Food mass is stored as energy, and then destroyed
3: Food mass is stored as energy, and then transformed into energy
4: Food mass is stored as energy, and then transformed into mass
5: Food mass is stored as mass, and then transformed into mass
6. Food mass is stored as mass, and then transformed into energy
Answers
Answer:
Food mass is stored as mass, and then transformed into energy
Explanation:
When we eat food, the mass of food undergoes the digestion process and is being converted to various molecules in the body. For instance carbohydrate foods are broken down into glucose which is now stored in the body as glycogen.
When energy is required in the body, this glycogen is broken down into glucose again and mass is converted to energy required to do work in the body.
how is the atomic radius calculated?
Answers
Explanation:
Divide the distance between the nuclei of the atoms by two if the bond is covalent
1. If a cell has waste it needs to store, it will store it:
Answers
Perform the following calculations and express the answers in
scientific notation.
a. 4.2 x 104 kg + 7.9 x 10³ kg
b. (5.23 x 106 um) (7.1 x 10-² μm)
c. 5.44 x 107 g + 8.1 x 104 mol
d. 8.40 x 105 km - 3.1 x 105 km
Answers
The answer of the calculations above in scientific notation are a=8.3 ×10³, b= 3.9 × 10^¹um, c= 1.4 × 10³gmol, d= 5.6× 10²km
Calculation to expressionsIn calculation of various expressions, the results can be expressed in a scientific notation which is a way to compress cumbersome figures.
(4.2 x 104 kg) + (7.9 x 10³ kg)4.2 × 104 = 436.8
7.9 × 10³ = 7900
436.8 + 7900 = 8,336.8
The scientific notation= 8.3 ×10³kg
(5.23 x 106 um) (7.1 x 10-² μm)5.23 x 106 um = 554.38um
554.38 × 7.1 = 3,936.1
3,936.1 × 10^-²
3.9 × 10³ × 10^-²= 3.9 × 10^¹um
The scientific notation= 3.9 × 10^¹um
5.44 x 107 g + 8.1 x 104 mol5.44 x 107 g = 582.08g
8.1 x 104 mol = 842.4 mol
582.08g+ 842.4 mol = 1,424.48
The scientific notation = 1.4 × 10³gmol
(8.40 x 105 km) - (3.1 x 105 km)8.40 x 105 km = 882km
3.1 × 105 = 325.5km
882km - 325.5km = 556.5
The scientific notation = 5.6× 10²km
Learn more about scientific notation here:
https://brainly.com/question/27862246
#SPJ1
A sample of helium gas has a pressure of 1. 20 atm at 295 K. At what temperature in Kelvin will the helium reach a pressure of 2. 00 atm, assuming the volume is constant?
Answers
The temperature in the Kelvin will the helium has when the pressure reaches of 2.00 atm, is 491 K.
The ideal gas equation is as :
P V = n R T
Where,
The pressure = P
The volume = V
The moles = n
The gas constant = R
The temperature = T
The pressure and the temperature relation is as :
P₁ / T₁ = P₂ / T₂
T₂ = P₂ T₁ / P₁
Where,
The pressure, P₁ = 1.20 atm
The temperature, T₁ = 295 K
The pressure, P₂ = 2 atm
The temperature, T₂ = ?
The final temperature is as :
T₂ = P₂ T₁ / P₁
T₂ = ( 2 × 295 ) / 1.20
T₂ = 491 K
The final temperature is 491 K.
To learn more about temperature here
https://brainly.com/question/29388783
#SPJ4
Which has more potential energy melted or solid
Answers
Molecules are not reacting using current methods. Which change can encourage a reaction?
O adding another chemical
O speeding up the molecules by mixing
O removing heat
O adding heat
Answers
Adding heat to a system can encourage or increases a chemical reaction.
Change that can encourage a reactionIncreasing the temperature of a process, increase occurs in the average speed of the reactant molecules and we know that when more molecules move faster, the more number of molecules reacts with other molecules which results in faster formation of products.
In conclusion, adding heat is the right option.
Learn more about reaction here: https://brainly.com/question/26018275
Which describes an effect that ocean currents have on short-term climate change? Ocean currents increase the strength of prevailing winds, which can cool the air and land. Ocean currents can carry cold water, which can cool the air and land. Ocean currents increase hurricane activity, which can raise the temperature of the air and land. Ocean currents carry cold water, which may not affect hurricane activity and lower the temperature of the air and land.
Answers
The movement of the ocean and sea waves in a direction is called the ocean currents. Ocean currents can bring cold water, which cools the land and atmosphere.
What are the effects of ocean currents?Ocean currents are the movement of the oceanic waves by the influence of the gravitational forces that can affect the atmosphere and the climate of the region.
Oceanic currents are capable of carrying cold water from polar areas to the regions of the equator resulting in a cooling atmosphere over the geosphere.
Therefore, option B. oceanic currents carry colder air to the warmer regions.
Learn more about oceanic currents here:
https://brainly.com/question/12174052
Answer:
B
Explanation:
Which of following is incorrect?
A. Beryllium oxide - Amphoteric nature
B. Potassium superoxide - Basic nature
C. Carbon monoxide - Acidic nature
D. Bismuth Oxide - Basic nature
Answers
Answer:
C: Carbon monoxide - Acidic nature
Explanation:
A) Beryllium is not soluble in water but yet it reacts with an acid and a base. Therefore, since it exhibits both basic and acidic nature, we say it is Amphoteric in nature. Thus it is correct
B)potassium super oxide has the formula KO2. It reacts with acid to form salt called potassium chloride. Thus, it is basic in nature.
C) Carbon monoxide does not react with acids or bases. Thus, it doesn't show either an acidic or basic property and we say it is a neutral oxide. This option is incorrect
D) Bismuth oxide is a basic oxide because it displays properties of a base. This options is correct
What does an isochron represent?
A) the age of a rock, given the composition of minerals
B) the rate of radioactive decay of elements
C) the ratios of all the different elements within a rock
D) the different ages of rocks, given a single mineral
Answers
An isochron represents the age of a rock, given the composition of minerals. In geochronology, isochron dating is a technique used to determine the age of rocks or geological samples.
Isochron dating relies on analyzing the ratios of isotopes within the minerals present in the rock. Isotopes are variants of an element with different numbers of neutrons in their atomic nuclei. By measuring the isotopic ratios, specifically the parent and daughter isotopes, scientists can calculate the time elapsed since certain geological events, such as the crystallization of the rock or a metamorphic event.
The isochron method involves plotting the isotopic ratios on a graph, typically using a set of minerals from the same rock sample. If the minerals formed at the same time, the data points will fall along a straight line known as an isochron. The slope of the isochron line provides the age of the rock, while the intercept with the y-axis indicates the initial isotopic composition. This technique helps to overcome challenges such as the presence of inherited isotopes or disturbances in the isotopic system, providing a reliable estimate of the rock's age.
To know more about isochron, click here, https://brainly.com/question/31478583
#SPJ11
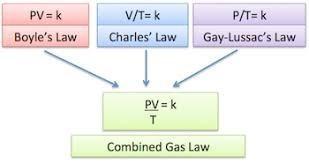
At 6 atmosphere pressure, the volume of a gas is 1200 ml. What pressure is
required to reduce the volume of a gas to 200 ml without changing the
temperature?
Answers
Answer:
36 atmExplanation:
The pressure required can be found by using the formula for Boyle's law which is
\(P_1V_1 = P_2V_2\)
Since we are finding the required pressure
\(P_2 = \frac{P_1V_1}{V_2} \\\)
From the question we have
\(P_2 = \frac{6 \times 1200}{200} = \frac{7200}{200} = \frac{72}{2} \\ \)
We have the final answer as
36 atmHope this helps you
In the Haber Process, ammonia is synthesized from nitrogen andhydrogen:
N2 (g) + 3H2 -----> 2NH3(g)
ΔG at 298K for this reaction is -33.3 kj/mol. the valuef ΔG at 298 K for a reaction mixture that consists of 1.9 atmN2, 1.6 atm H2 and 0.65 atm NH3 is________.
a.) -3.86 x 103
b.) -1.8
c.) -7.25 x 103
d.) -40.5
e.) -104.5
Answers
The value of ΔG at 298 K for a reaction mixture containing 1.9 atm N2, 1.6 atm H2, and 0.65 atm, the answer is (a) -3.86 × 10^3.
NH3 can be calculated using the equation:
ΔG = ΔG° + RT ln(Q)
where ΔG is the standard Gibbs free energy change, ΔG° is the standard Gibbs free energy change at standard conditions, R is the gas constant, T is the temperature in Kelvin, and Q is the reaction quotient.
In this case, we are given ΔG° as -33.3 kJ/mol. To calculate Q, we need to use the partial pressures of the gases in the reaction mixture. The reaction stoichiometry tells us that the ratio of the partial pressures of N2, H2, and NH3 is 1:3:2. Therefore, we can write:
Q = (P(NH3))^2 / (P(N2) * P(H2)^3)
Plugging in the given values of P(N2) = 1.9 atm, P(H2) = 1.6 atm, and P(NH3) = 0.65 atm, we can calculate Q. Then, using the value of R = 8.314 J/(mol·K) and the temperature T = 298 K, we can substitute these values into the equation and solve for ΔG.
The calculated value of ΔG at 298 K for the given reaction mixture is approximately -3.86 × 10^3 J/mol. This value is equivalent to -3.86 kJ/mol. Therefore, the answer is (a) -3.86 × 10^3.
To learn more about Haber Process here brainly.com/question/30928282
#SPJ11
An ionic compound is
A.a salt
B.held together by a transfer of electrons
C.composed of anions and cations
D.held together by sharing of valence electrons
Answers
which is larger Ca or Ca2+
Answers
Answer:
Ca
Explanation:
Ca is larger than Ca2+ because, in the periodic table, the atomic radius increases from top to bottom and from left to right. Also Ca has 2 more electrons than Ca2+ therefore, its atom is bigger because it has an extra shell.
Ca²⁺ is smaller than Ca. As Ca²⁺ has lost two electrons to become positively charged.
Ca²⁺ is the calcium ion, which has lost two electrons to become positively charged. When an atom loses electrons, it becomes smaller because there are fewer negatively charged electrons in the electron cloud to balance the positive charge of the nucleus.
On the other hand, Ca refers to the neutral calcium atom, which has an equal number of protons and electrons. In comparison to Ca²⁺, the neutral calcium atom has two additional electrons, which increases the electron cloud and overall size of the atom.
Therefore, Ca²⁺ is smaller than Ca.
Learn more about calcium here:
https://brainly.com/question/30954368
#SPJ 4
HELPPPPPPPPP!!!!....... Which statement best describes how chemical equations demonstrate conservation of mass?
A.
The number of reactants is the same as the number of products.
B.
The compounds are the same on each side of the reaction.
C.
The number of atoms of each element is the same on each side of the equation.
D.
The state of matter is the same on each side of the equation.Which details from the passage are emphasized in both the text and images?
Answers
Answer:
A. The mass of reactants is the same as the mass of products
are bonds stronger or weaker when they are longer? justify your reasoning using the principles of coulombic attraction.
Answers
Two factors determine how strong the coulombic attraction is: the dimensions of an atom. the atom's overall charge.
Why do smaller ions coulombically attract water more strongly than larger ions?
using the law of Coulomb. The water molecules find it more difficult to separate the ions from one another the smaller the ions are and the closer together they are. The more charged the ions are, the more difficult it is for the water to also remove them.
How does the size of an atom change due to coulombic attraction?
The atomic radius will shrink when the valence electrons are drawn in closer to the nucleus due to a higher force of attraction between the nucleus and the valence electrons.
To know more about coulombic attraction vist:
https://brainly.com/question/15173422
#SPJ4
You're going on a plane from Alaska to Florida and you're taking your bike. It's the middle of the winter. You fill up your tires before you leave to a pressure of 2.72
atm on a day that is -7.50°C. When you arrive in FL at 38°C, you check the pressure in your tires before you ride, what do you find the pressure to be?
Type your answer with the proper number of significant figures and proper units in the space below.
Answers
Answer:
2.3 atm
Explanation:
-7.50°C = 265.5 K
38°C = 315 K
(2.72)(265.5)=(pressure)(315)
pressure = 2.3 atm