What is the freezing point of a solution of 210.0 g of glycerol, formula C3H8O3, dissolved in 350. g of water? Careful. First get molar mass and use molar mass to determine molality concentration. Then use freeze pt. depression formula to determine the change in freezing pt. Then determine the new freeze point. The freezing point depression constant for water is Kf= -1.86 oCelcius/molal. Report your answer rounded to 1 decimal point and do not include units.
Answers
Answer:
- 12.1
Explanation:
M(C3H8O3) = 3*12.0 + 8*1.0 + 3*16.0 = 92 g/mol
210.0 g C3H8O3 * 1 mol C3H8O3/92 g C3H8O3 = 210/92 mol C3H8O3
350.g = 0.350 kg H2O
Molality = mol soluty/ kg solvent = (210/92 mol C3H8O3) /(0.350 kg H2O) = =6. 522 molal
ΔT =i* Kf* m
(T2 - T1) = i* Kf* m
(T2 - 0°C) = 1*(-1.86°C/molal *6.522 molal)
T2= - 12.1°C
The change in freezing point and the new freeze point are -12.1°C and -12.1°C.
Given the following data:
Mass of glycerol = 210.0 gramsMass of water = 350.0 grams to kg = 0.35 kgChemical formula of glycerol = \(C_3H_8O_3\)Freezing point depression constant for water, Kf = 0.512 °C/mWe know that the temperature at which water freezes is 0°C.
To determine the change in freezing point and the new freeze point:
First of all, we would determine the molar mass of glycerol:
Molar mass of glycerol (\(C_3H_8O_3\)) = \(12 \times (1 \times 8)\times (16 \times 3)\)
Molar mass of glycerol (\(C_3H_8O_3\)) = \(12 \times (8)\times (48)\)
Molar mass of glycerol (\(C_3H_8O_3\)) = 92 g/mol.
Next, we would find the number of moles of glycerol that is required:
\(Number\;of\;moles = \frac{mass}{molar\;mass}\\\\Number\;of\;moles = \frac{210.0}{92}\)
Number of moles = 2.28 moles
To find the molality concentration:
\(Molality = \frac{moles\;of \;solute}{mass\;of \;solvent} \\\\Molality = \frac{2.28}{0.35}\)
Molality = 6.51 molal.
Mathematically, the freezing point elevation of a liquid is given by the formula:
\(\Delta T = K_f m\)
Where:
\(\Delta T\) is the change in temperature. Kf is the molal freezing point constant. m is the molality of solution.
Substituting the parameters into the formula, we have;
\(\Delta T = -1.86 \times 6.51\)
Change in temperature = -12.1°C.
Now, we can determine the new freeze point:
\(\Delta T = T_n - T_f\\\\T_n = \Delta T + T_f\\\\T_n = -12.1 + 0\)
New freeze point = -12.1°C.
Read more: https://brainly.com/question/13750908
Related Questions
2. Several organs make up the excretory system, including the lungs, skin,
kidneys
and
Answers
Answer:
large intestine
Explanation:
The large intestine's job is to dehydrate what's left of the food and form it into stool. It accomplishes this by gradually absorbing electrolytes and water while its muscular system transfers the waste. While this is happening, bacteria in your large intestine feed on the waste and further decompose it, completing the chemical phase of digestion.
Question
The weak base pyridine, C5H5N, is added to water to make up a solution of 0.248 M pyridine in water. The Kb of pyridine
is 1.7 x 109. What is the pH of the solution?
• Round your answer to two decimal places.
Sorry, that's incorrect. Try again?
11.56
Answers
The pH of the solution is approximately 4.84.
To find the pH of the solution, we need to calculate the concentration of hydroxide ions (OH-) and then convert it to the pH scale.
First, let's determine the concentration of hydroxide ions (OH-) using the Kb value and the concentration of pyridine (C5H5N).
Kb = [OH-][C5H5N] / [pyridinium ion]
Since pyridine is a weak base, we can assume that the concentration of pyridinium ion is negligible compared to the concentration of pyridine. Therefore, we can simplify the equation to:
Kb = [OH-][C5H5N]
Now, we can rearrange the equation to solve for [OH-]:
[OH-] = Kb / [C5H5N]
Substituting the given values, we have:
[OH-] = (1.7 x 10^9) / (0.248)
[OH-] ≈ 6.85 x 10^9
Now that we have the concentration of hydroxide ions, we can calculate the pOH:
pOH = -log10([OH-])
pOH = -log10(6.85 x 10^9)
pOH ≈ 9.16
Finally, we can calculate the pH using the relationship between pH and pOH:
pH = 14 - pOH
pH ≈ 14 - 9.16
pH ≈ 4.84
For more such questions on pH visit:
https://brainly.com/question/12609985
#SPJ8
2.59 Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F, (b) Li and H, (c) Al and I, (d) K and S.
Answers
Answer:
(a) GaF3, gallium(III) fluoride
(b) LiH, lithium hydride
(c) AlI3, aluminum(III) iodide
(d) K2S, potassium sulfide
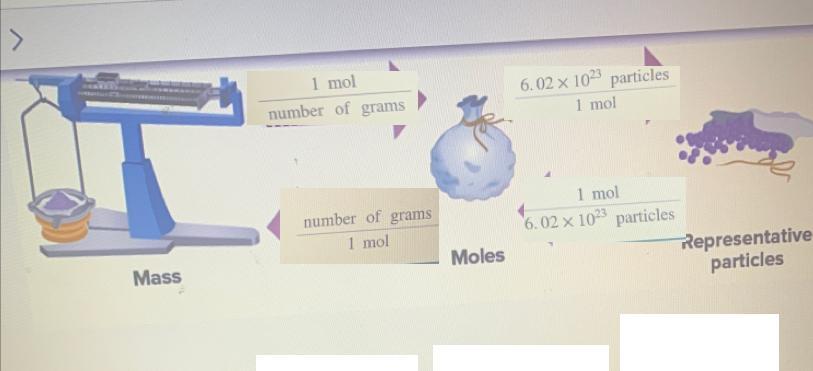
Drag each conversion factor to the arrow that indicates where it should be used

Answers
Explanation:
mass ----> moles ---> representative particles:
To go from mass to moles we usually use the molar mass of the substance or compound that we are working with. Molar mass is expressed in g/mol, so we divide by the molar mass.
mass --> moles: g * 1 mol/(number of g) = mol
To go from moles to representative particles we use Avogadro's number. There are 6.02 *10^23 particles in 1 mol of particles.
moles ---> particles: moles * 6.02 * 10^23 particles /(1 mol) = particles
Then to go from particles to moles we do something similar.
particles---->moles: particles * 1 mol/(6.02 * 10^23 particles) = moles
And to go from moles to grams, instead of dividing by the molar mass we multiply by it.
moles --> mass: moles * number of g/(1 mol) = g
Answer:
mass ----> 1 mol/(number of g) ---> moles ---> 6.02 * 10^23 particles /(1 mol) ----->particles
mass <---number of g/(1 mol) <----- moles <----1 mol/(6.02 * 10^23 particles)<----- particles
A student mixes some soap with water and then blows through a straw into the solution. Bubbles form. Do you think a chemical change has taken place? Explain
Answers
Answer:
No it has not.
Explanation:
The actual chemical compound has not changed, the materials have mixed but no reaction has taken place. Blowing the bubbles causes a physical change. Hope this helped!
Mixing soap and water and forming bubbles is a physical change as there is no change in composition.
What is a physical change?
Physical changes are defined as changes which affect only the form of a substance but not it's chemical composition. They are used to separate mixtures in to chemical components but cannot be used to separate compounds to simpler compounds.
Physical changes are always reversible using physical means and involve a change in the physical properties.Examples of physical changes include melting,boiling , change in texture, size,color,volume and density.Magnetism, crystallization, formation of alloys are all reversible and hence physical changes.
They involve only rearrangement of atoms and are often characterized to be changes which are reversible.
Learn more about physical changes,here:
https://brainly.com/question/17931044
#SPJ2
Explain the difference between repetition and replication.
Answers
Answer:
Repetition happens when measurements are taken during the same experimental run , which means that the same person runs the experiment in multiple trials . on the other hand , replicayion occurs when an experiment is reproduced by different experimental run .
Answer:
Repetition refers to performing multiple trials throughout an experiment. Repetition reduces mistakes and increases one’s confidence in the results. Replication refers to the ability of a process to be repeated by another individual. When a scientist replicates the experiment of another, the experiment should produce the same results.
Explanation:

what is the example of the theory of needs by jean Baptiste de lamark
Answers
Answer: Jean Baptiste de Lamarck is a famous biologist known for his theory of evolution. While he is known for his theory of inheritance of acquired characteristics, he also proposed a theory of needs to explain the evolutionary process.
According to Lamarck's theory of needs, animals have specific needs that arise due to changes in their environment. For example, if an animal lives in an area with tall trees, it may need to stretch its neck to reach the leaves for food. Over time, Lamarck believed that this need would cause the animal's neck to lengthen, and this acquired trait would be passed down to its offspring.
One example of Lamarck's theory of needs can be seen in the evolution of the giraffe. Lamarck proposed that the giraffe's long neck evolved due to a need to reach high branches for food. According to his theory, over time, the giraffe's neck lengthened as a result of this need, and this acquired trait was passed down to future generations, eventually resulting in the long-necked giraffes we see today.
However, it is important to note that Lamarck's theory of needs has been largely discredited in modern evolutionary theory, which relies on the principles of natural selection and genetic mutation to explain the process of evolution.
Explanation: i would reallyyyyyyy apreciate brainliest
19
Provide the correct name for this molecule:

Answers
Answer:
Nihonium
Explanation: it's atomic number is 113 and It's a radioactive element
Explain why you think it is important to know the three different types of rocks.
Answers
Answer:
because they contains clues about what the earth was like in the past. different rocks from under only certain conditions and even the dullest gray lump of rock.
Why was the morning session stopped unsuccessfully?
The reactor was overheating.
The automatic control system was not adjusted properly.
The vernier control rod became stuck.
The emergency rod failed.
Answers
Answer:
the reactor was overheating
what is density? chemistry
Answers
Answer: Density means the compactness of an object
I hope this helps, and Happy Holidays! :)
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. According to kinetic-molecular theory, which of the following statements is true? Check all that apply. If the temperatures of both containers are equal, container A has greater pressure than container B. If the volume of container A decreased, its pressure would decrease. If the pressure in both containers is equal, container A has a lower temperature than container B. Two containers are shown. Container A is square, and Container B is the same height, but is about twice as wide. Each container holds 6 gas particles distributed randomly.
Answers
The kinetic-molecular theory's true assertions are as follows:
If both containers' temperatures are the same, container A will have a higher pressure than container B.
Container A has a lower temperature than container B if the pressure in both containers is equal.
What is the kinetic theory of molecules?The molecules that make up a gas are always moving randomly, colliding with one another and the container walls, according to the kinetic molecular hypothesis. Remember that high temperature and low pressure are the only conditions in which perfect gases can exist.
The kinetic-molecular theory's true assertions are as follows:
If both containers' temperatures are the same, container A will have a higher pressure than container B.
If both are under pressure
Learn more about kinetic molecular theory:brainly.com/question/12025712
#SPJ1
Would the chlorine atom or ion be larger?
Answers
Answer:
Chlorine Ion
Explanation:
The chlorine ion is adding 1 valence electron in order to complete its outer most shell. It has a charge of 1- meaning it is adding an electron.
The radius of a nickel atom is 125 pm. How many nickel atoms would have to be laid side by side to span a distance of 1.74 mm
Answers
Answer:
6960000 Nickel atoms
Explanation:
radius = 125 pm = 125 x 10^-12 m
diameter of each atom = 125 pm x 2 = 250 pm = 250 x 10^-12 m
span of distance to be achieved = 1.74 mm = 1.74 x 10^-3 m
number of nickel atoms = (1.74 x 10^-3 m)/(250 x 10^-12 m) = 6960000 Nickel atoms
A constant electric current deposited 365 mg of Ag in 216 minutes from an aqueous Silver trioxonitrate (v). What is the Current?
Answers
The electric current is 0.025 A
Electric current refers back to the go with the flow of energy in an electronic circuit and to the amount of strength flowing through a circuit. it's far measured in amperes (A). the bigger the cost in amperes, the more energy is flowing within the circuit.
Ag+ + e¯ →Ag
1F deposits 107.87 g/mol (molecular mass) of silver
1F = 96500 C
Let, 107.87 g/mol needed = 96500 C
Number of coulombs required to deposit 0.3650 g of silver =(96500/107.87) 0.3650
Q = 326.5 C
According to Faraday’s law, Q = I x t
I = 326.5 C / (216 x 60 s) = 0.025 A
Learn more about electric current here:-https://brainly.com/question/2984202
#SPJ9
john dalton law of partial pressure
Answers
According to Dalton's law of partial pressures, the total pressure by a mixture of gases is equal to the sum of the partial pressures of each of the constituent gases. The partial pressure is defined as the pressure each gas would exert if it alone occupied the volume of the mixture at the same temperature. The Law of Partial Pressures is commonly applied in looking at the pressure of a closed container of gas and water. The total pressure of this system is the pressure that the gas exerts on the liquid. The gas is made up of whatever sample of gas there is plus the evaporated water. Dalton's law of partial pressures, Pt = P1 + P2 + ..., says that the total pressure of a gas mixture is the sum of the partial pressures of constituent gases. Dalton's law states that the total pressure of a gas is the sum of the individual pressures of all the gas molecules in the container. In other words, it's the average pressure exerted by all the gas particles in a given system.
I need help on 9, 10, 11, and 12

Answers
The limiting reactant and excess reactant can be determined by a concept known as the Law of Conservation of Mass.
The limiting reactant and excess reactantLimiting Reactant: H2O, Excess Reactant: O2Limiting Reactant: Mg(OH)2, Excess Reactant: HCILimiting Reactant: Fe2O3, Excess Reactant: ZnLimiting Reactant: CuSO4, Excess Reactant: AgNO3This law states that matter can neither be created nor destroyed. Therefore, the amount of each reactant must be equal to the amount of each product. By looking at the chemical equation and the amount of each reactant, the limiting reactant is the one that is used up first and therefore determines the amount of products that can be made. The excess reactant is the one that is left over once the limiting reactant has been exhausted. For example, in problem 9, the equation is 2H2O + O2 →→ 2H2O2 and the amounts are 5 mol H2O and 15 mol O2. Since oxygen is used up first, it is the limiting reactant and hydrogen is the excess reactant. In problem 10, the equation is Mg(OH)2 + 2HCI→→ MgCl2 + 2H2O and the amounts are 6 mol Mg(OH)2 and 20 mol HCI.Since magnesium is used up first, it is the limiting reactant and hydrochloric acid is the excess reactant. In problem 11, the equation is 3Zn + Fe2O3→ 3ZnO + 2Fe and the amounts are 4 mol Zn and 2 mol Fe2O3. Since zinc is used up first, it is the limiting reactant and iron oxide is the excess reactant. In problem 12, the equation is CuSO4 + 2AgNO3 → Cu(NO3)2 + Ag₂SO4 and the amounts are 13 mol CuSO4 and 22 mol AgNO3. Since copper sulfate is used up first, it is the limiting reactant and silver nitrate is the excess reactant.To learn more about The limiting reactant and excess reactant refer to:
https://brainly.com/question/14222359
#SPJ1
Identify an element on the periodic table that is chemically similar to boron (B)
Options
CI
Si
Ga
Ds
Os
Mo
Ti
Li
(PLEASE HURRY ASAP IM ON A PLATO MASTERY TEST!) (Image shows the periodic table and options)

Answers
Answer:
Silicon
Explanation:
Hope this helps! :)
Answer: Ga
Explanation: I got 5/5 correct on my mastery test :)
Brian's aunt has cats. When Brian recently visited her, he started sneezing badly and believes that it was because
he is allergic to cats.
What would be the next step for Brian to form a hypothesis?
O by avoiding going to his aunt's house
by wearing a nose mask near cats
by visiting other households with cats
O by visiting his aunt's house again with the cats outside
Answers
Answer:
By visiting other households with cats.
Explanation:
This will give Brian a variety of other houses and determine if it is truly cats or just alleries from other items. This is the most direct way to get Brian the answer he is looking for.
Answer:
c
Explanation:
i just did it
Which correctly lists the three weather factors that are indicators of climate change?
a.) sunsets, wind patterns, clouds
b.) ocean currents, ice cores, temperature
c.) temperature, wind patterns, ice cores
d.)wind patterns, temperature, ocean currents
Answers
Answer:
C
Explanation:
c.) temperature, wind patterns, ice cores
How many total ions are there in 5.00 moles of cobalt (II) bromide?
Answers
PLEASE HELP NOW
Caffeine is a weak base with a b of 4.1×10^-4 Calculate the initial molar concentration of a solution of caffeine if the pH is 10.94.
Answers
Answer:430 mg/L = 0.43g/L
Explanation:
Is cooking an egg a chemical reaction
(I will give brainiest)
Answers
Answer:
cooking an egg is a chemical reaction because you can change the cooked egg into it original form
Explanation:
pls pls give brainliest pls
Answer:
Yes,Cooking an egg is a chemical reaction.
Explanation:
Cooking an egg is a chemical reaction because you can't change cooked egg into raw or its previous state.There are two types of reactions
Physical reactionChemical reactionbruh i need help and someone just used the last one for points plz help
Answers
Answer: 5000
Explanation:
Hãy cho biết giá trị và ý nghĩa của số lượng tử n, l, m, ms khi mô tả trạng thái của electron trong nguyên tử?
Answers
In the following unbalanced combustion reaction how many grams of C8H18 will react with 24.78g of O2.

Answers
7.10 grams of C₈H₁₈ will react with 24.78 g of O₂ in this unbalanced combustion reaction.
What is meant by combustion reaction?Type of chemical reaction that occurs when substance reacts with oxygen gas to produce energy in the form of heat and light is called combustion reaction .
To balance the combustion reaction: C₈H₁₈ + 12.5 O₂ -> 8 CO₂ + 9 H₂O
The coefficients show that 12.5 moles of O₂ are needed to react with 1 mole of C₈H₁₈.
1 mole of O₂ has a mass of 32 g, so 24.78 g of O₂ is:
24.78 g / 32 g/mol = 0.774 mol of O₂
0.774 mol of O₂ / 12.5 mol of O₂/mol of C₈H₁₈ = 0.06192 moles of C₈H₁₈
0.06192 moles of C8H18 x 114.23 g/mol of C₈H₁₈ = 7.10 g of C₈H₁₈
Therefore, 7.10 grams of C₈H₁₈ will react with 24.78 g of O₂ in this unbalanced combustion reaction.
To know more about combustion reaction, refer
https://brainly.com/question/13251946
#SPJ1
Calculate the volume of chlorine molecules produced at room temperature and pressure, when 234g of sodium chloride are electrolysed. (1 mole of chlorine molecules has a volume of 24 dm³ at room temperature and pressure).
Answers
The volume of chlorine molecules produced at STP would be 96 dm³.
Stoichiometric problemSodium chloride ionizes during electrolysis to produce sodium and chlorine ions as follows:
\(NaCl --- > Na^+ + Cl^-\)
This means that 1 mole of sodium chloride will produce 1 mole of sodium ion and 1 mole of chlorine ion respectively.
Recall that: mole = mass/molar mass
Hence, 234 g of sodium chloride will give:
234/58.44 = 4.00 moles.
Thus, the equivalent number of moles of chlorine produced by 234 g of sodium chloride will be 4 moles.
Recall that:
1 mole of every gas at Standard Temperature and Pressure = 24 Liters.
Hence:
4 moles of chlorine = 4 x 24 = 96 Liters or 96 dm³.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
Please help me thanks so much?!?!

Answers
Answer:
D.
Explanation:
I did this question in school and got it right.
Answer:
The answer to your question is when the size, shape, or state of matter changes.
Explanation:
Change that does not undergo in the chemical composition.
What is the balanced form of the chemical equation shown below?
Ca(OH)2(aq) + Na2CO3(aq) → CaCO3(s) + NaOH(aq)

Answers
Answer:
D
Explanation:
Double Displacement reaction
Both sides are balanced with option D
The balanced form of the chemical equation shown below is \(\rm Ca(OH)_2(aq) + Na_2CO_3(aq) \rightarrow CaCO_3(s) + 2NaOH(aq).\) The correct option is D.
What is a balanced equation?A balanced equation is where the reactant and the product have the number of moles of elements. According to the law, the reaction, and the product have the same number of moles after the reaction, so balancing an equation is important.
To balance an equation, it is significant to see the number of moles of reactant and the same number of moles is in the product side. Here the moles of sodium has to be balanced.
Thus, the correct option is D, \(\rm Ca(OH)_2(aq) + Na_2CO_3(aq) \rightarrow CaCO_3(s) + 2NaOH(aq).\)
Learn more about the balanced equation, here:
https://brainly.com/question/12192253
#SPJ5