what is relative abundance isotopes
Answers
The relative abundance of isotopes is the number of atoms of a particular isotope divide by the total number of atoms of all isotopes of that element, multiplied 100 percent.
What is relative abundance isotopes?The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
Also relative abundances refers to the relative proportions of the stable isotopes of each element. They are most often quoted as atom percentages
To calculate the percent abundance of each isotope in a sample of an element, the number of atoms of a particular isotope is usually divide by the total number of atoms of all isotopes of that element and then multiply the result by 100 since it is expressed in percentage.
Mathematically, the formula for relative abundance is given as;
R.A = ( number of atoms of isotope / total number of atoms ) x 100%
Learn more about relative abundance here: https://brainly.com/question/6844925
#SPJ1
Related Questions
Which statement best explains why aluminum is a pure substance? Group of answer choices Aluminum is one type of atom and its composition is always the same. Aluminum has a composition that is evenly mixed. The aluminum atom cannot be seen with a microscope. Aluminum contains substances that are not bonded together.
Answers
Answer:
The best explanation for both the first and second claims is that aluminum is a pure substance: Aluminum is one form of atom and its structure is always the same. Aluminum has an evenly blended composition.
Explanation:
Aluminum is one type of atom and its composition is always the same, this statement also gives the property of a pure substance . In fact, pure substances are often homogeneous and contain only one form of atom or molecule.
One of a pure substance's key characteristics is that it has a uniform composition that is homologous in nature, and can be mixed uniformly throughout. ALUMINUM's second statement notes the property of a pure substance. Aluminum is also homologous in terms of occurrence.
The atoms are very small and have a diameter of around 1 x 10-10 meters. It's difficult to view them using a light microscope due to their limited size. Although it may not be possible to view an atom using a light microscope, a number of techniques have been developed, such as electron microscopy, to observe and research the structure of atoms. Therefore, the statement that the aluminum atom can not be seen through a microscope does not explain the pure content of aluminum.
This argument - 'Aluminum contains substances which are not bound together' does not explain the fact that it is a pure material as it notes that the property of a pure material does not exist in relation to its chemical bonds.
.Therefore, the first and second aluminum statements give the best description of a pure material.
a certain carbohydrate produced an observed rotation of 2.70 degrees at a concentration of 0.022 g/ml in a 1 dm cell. what is its specific rotation? (do not include any units.)
Answers
The specific rotation of the carbohydrate is 12.27 when observed rotation is 2.70 degrees at a concentration of 0.022 g/ml in a 1 dm cell
The specific rotation, denoted by [α], is a measure of the ability of a compound to rotate plane-polarized light and is defined as the observed rotation in degrees divided by the concentration in grams per milliliter and the path length in decimeters.
Mathematically, we can express it as:
[α] = observed rotation / (concentration x path length)
In this problem, we are given:
observed rotation = 2.70 degrees
concentration = 0.022 g/mL
path length = 1 dm = 10 cm
Substituting these values into the formula, we get:
[α] = 2.70 degrees / (0.022 g/mL x 10 cm)
[α] = 2.70 degrees / 0.220 g/cm³
[α] = 12.27
Learn more about the specific rotation here.https://brainly.com/question/18523033
#SPJ11
Chalk is a silicate carbonate evaporite sandstone QUESTION 33 a photosyntehtic creature with a silica shell can be a O coccolithophorid foraminifer diatom radiolarian QUESTION 34 recrystallization of chalk at the ocean bottom (not in metamorphic conditions) can give us O micrite chert marble quartzite
Answers
Diatoms are single-celled algae that have a silica (silicate) shell called a frustule.
Diatoms are photosynthetic organisms and are known for their intricate and diverse shapes. Diatoms are commonly found in freshwater and marine environments and play a significant role in the global carbon cycle.
Micrite is a fine-grained carbonate sedimentary rock composed of tiny carbonate particles. It forms through the precipitation and accumulation of carbonate minerals, such as calcite or aragonite, in marine environments. In the case of chalk, which is primarily composed of microscopic fragments of calcium carbonate from marine organisms, recrystallization can occur at the ocean bottom under specific conditions, leading to the formation of micrites.
Therefore, it's important to note that chert, marble, and quartzite are not the typical products of recrystallization of chalk at the ocean bottom.
For more details regarding diatoms, visit:
https://brainly.com/question/11446176
#SPJ4
When carbon is completely combusted in the presence of oxygen the only product produced is carbon dioxide as shown in the following equation, C+O2——>CO2 If 12 grams of carbon are burned, how many grams of carbon dioxide will be produced?

Answers
Answer:
C.44g is that one okkkkkkkkkkkkkkkk
A total of 1.436 F of electricity (1 F=1 mol e−) was required to electrodeposit all of the Zn and Co from a solution of ZnSO4 and CoSO4. The mixture of Zn and Co that was deposited had a mass of 43.57 g. Calculate the masses of ZnSO4 and CoSO4 present in the original solution.
Answers
There were approximately 128.94 g of ZnSO4 and 109.34 g of CoSO4 present in the original solution.
What is electroplating?Electroplating is the process of coating a metal object with a thin layer of another metal by means of electrolysis. In an electrolytic cell with a solution of a salt of the metal to be deposited, the item to be plated is made the cathode (negative electrode).
The electroplating of Zn and Co from the solution involves the transfer of electrons from the cathode to the metal ions in the solution, which results in the deposition of the metals on the cathode. The amount of electricity required for this process is proportional to the amount of metal ions present in the solution, which in turn is proportional to the mass of the metals deposited.
Let's first calculate the moles of electrons transferred in the electroplating reaction:
1.436 F × (1 mol e⁻/1 F) = 1.436 mol e⁻
Since the number of electrons transferred is the same for both Zn and Co, the ratio of the moles of Zn and Co deposited should be the same as the ratio of their atomic masses. The atomic masses of Zn and Co are 65.38 g/mol and 58.93 g/mol, respectively, so the ratio of their masses is:
65.38 g/mol ÷ 58.93 g/mol ≈ 1.11
This means that for every 1.11 moles of Zn deposited, 1 mole of Co is deposited.
Let's assume that x moles of ZnSO4 and y moles of CoSO4 were present in the original solution. Then we can set up the following equations based on the balanced electroplating reaction:
2 e⁻ + Zn²+ → Zn (s)
2 e⁻ + Co²+ → Co (s)
The total number of moles of electrons transferred in the electroplating reaction is:
1.436 mol e⁻ = 2 mol e⁻/mol Zn × x mol ZnSO4 + 2 mol e⁻/mol Co × y mol CoSO4
Simplifying and solving for y:
y = (1.436 mol e⁻ - 2 mol e⁻/mol Zn × x mol ZnSO4) / (2 mol e⁻/mol Co)
y = 0.718 mol CoSO4
Since the ratio of the moles of Zn to Co deposited is 1.11, we can calculate the moles of ZnSO4 from the moles of CoSO4:
x = (1.11 mol Zn/mol Co) × (0.718 mol CoSO4) = 0.798 mol ZnSO4
Finally, we can calculate the masses of ZnSO4 and CoSO4:
mass of ZnSO4 = 0.798 mol × 161.47 g/mol = 128.94 g
mass of CoSO4 = 0.718 mol × 152.06 g/mol = 109.34 g
To know more about cathode, visit:
https://brainly.com/question/4052514
#SPJ1
Help would be greatly appreciated!
Which process requires the absorption of energy
Deposition
freezing
vaporization
condensation
Answers
Answer:
The process that requires the absorption of energy is vaporization. Vaporization is the process of changing a substance from a liquid to a gas. This requires energy, as the molecules must be given enough energy to break the bonds that hold them together in the liquid state. This energy is absorbed from the environment, causing the temperature of the surroundings to decrease.
Explanation:
give me brainiest
Answer: Vaporization.
Explanation: A process that requires the absorption of energy is vaporization. Vaporization is the conversion of a substance from a liquid or solid phase into a vaporous or gaseous phase. Energy is needed to break the bonds from the solid or liquid and change it to a gas.
Hope this helps! :)
Working from the sun outwards, which planet is the first to have at least one moon?
Earth
Venus
Mars
Mercury
Answers
Sun is the centre of the solar system containing 8 planets. Some planets have moons revolving around them. The planet from the sun which a moon is Earth.
What are planets?Planets are spatial objects formed by gases, soil, and dust and are having a gravitational force. The only living planet is earth. The moon of earth is itself called moon which is revolving around the earth.
In solar system the nearest planet to sun is mercury which have no moons. Next is venus also having no moons. The next planet in the series is earth having one moon.
After earth, mars is closest to sun which have two moons namely Deimos and Phobos. After mars,Jupiter having the highest number of moons and then saturn.
Therefore, the first planet from sun having at least one moon is earth.
To find more about solar system, refer the link below:
https://brainly.com/question/18365761
#SPJ1
Answer:
Explanation:
Answer:,,,,,
ATP, the energy used inside a cell, is created in the Ribosomes
?true or false?
Answers
Answer:
false
Explanation:
because its made in the mitochondria
which process is a chemical change
Answers
Determine the Activity (A) of a sample containing 4.076×10
20
atoms of
18
F. The half-life of
18
F is 109.77 min. Calculate and enter the intermediate result(s) as requested. Calculate the decay constant (k) for this isotope, with time units the same as those given for half-life. Report the result in scientific notation. Enter the value in the first blank, the exponent in the second blank, and the units in the second blank. Use negative exponents for units as necessary. k: ×10 Calculate the Activity (in Bq). Report your answer in scientific notation, with proper sig figs. Enter the value in the first blank and the exponent in the second blank.
Answers
To determine the activity (A) of the sample containing 4.076×10^20 atoms of ^18F, we need to calculate the decay constant (k) and then use it to calculate the activity. The activity of the sample is approximately 2.582×10^18 Bq.
The decay constant (k) can be found using the formula:
k = ln(2) / t1/2
where t1/2 is the half-life.
Using the given half-life of ^18F, which is 109.77 min, we can calculate the decay constant:
k = ln(2) / 109.77 min
Calculating this value gives us:
k ≈ 0.006322 min^-1
Next, we can calculate the activity (A) using the formula:
A = λ * N
where λ is the decay constant and N is the number of radioactive atoms.
Plugging in the values:
A = (0.006322 min^-1) * (4.076×10^20 atoms)
Calculating this gives us:
A ≈ 2.582×10^18 Bq
Therefore, the activity of the sample is approximately 2.582×10^18 Bq.
To learn more about decay constant click here
https://brainly.com/question/30330344
#SPJ11
A student is weighing a small amount of product, and is having issues with recording a correct mass. Which of the following could explain why this may be occurring? a) They are utilizing a different balance each time b) It is not possible to weigh very small masses c) Small masses must be weighed with a special scale only the TA can use d)None of the above
Answers
Answer: D) none of the above
Explanation:
The student might be inconsistent in using different balances when weighing the small product. Using a well-calibrated microbalance and the same one every time can help in attaining accurate measurements of small masses.
Explanation:The student's difficulty in accurately weighing a small amount of product could be due to option a) They are utilizing a different balance each time. This could introduce errors due to inconsistencies in the calibration and accuracy of different balances. However, it is not accurate that it is impossible to weigh very small amounts (option b) or that only a special scale that only the TA can use is needed (option c). Small masses can be weighed accurately with a well-calibrated microbalance. These instruments are designed to accurately measure small weights and are often found in laboratory settings. It is also crucial to use the same balance each time to ensure consistent results.
Learn more about Weighing Small Masses here:https://brainly.com/question/24508450
#SPJ2
How to write ionic compund formulas
ex Na+ & F-
Answers
You with writing ionic compound formulas. An ionic compound consists of a positive ion (cation) and a negative ion (anion) bonded together through electrostatic forces. To write the formula of an ionic compound, you need to balance the charges of the cation and anion to ensure the compound is neutral.
In your example, you have a sodium ion (Na+) and a fluoride ion (F-). The sodium ion has a positive charge of +1, while the fluoride ion has a negative charge of -1. To write the formula for the ionic compound formed by these two ions, you simply combine them in a way that balances their charges. Since the charges are already equal and opposite, you just need to put them together:
Na+ & F- → NaF
The resulting ionic compound is sodium fluoride (NaF). To write formulas for other ionic compounds, follow the same process:
1. Identify the cation and anion involved.
2. Determine the charges of each ion.
3. Balance the charges by adjusting the number of ions as needed.
4. Write the formula, placing the cation first followed by the anion.
Remember to always ensure that the charges are balanced, and the resulting compound is neutral. This method will allow you to write ionic compound formulas effectively.
Learn more about ionic compound here
https://brainly.com/question/28759239
#SPJ11
pls helppp if possible

Answers
The new volume of an ideal gas is 5935.7 L. The volume of 23g neon is 1139.01 L. The pressure in Torr is 26537 T. Temperature in Celsius is 3483°C. The volume of the balloon will be 1121.7 L.
An ideal gas is a type of gas in which molecules do not attract or repel each other. They only interact through elastic collision. An ideal gas equation is the equation for ideal gas molecules.
Mathematically it is represented as :
PV = nRT
where:
P = Pressure in atm.
V = Volume in atm.
n = Number of moles.
R = Universal Gas Constant.
T = Temperature in Kelvin.
Given:
5. T = 100°C ( Converting into Kelvin : 100 + 273 = 373 K)
P = 1.4 atm
By substituting the values in the above-given equation:
V = 1 × 8.314 × 373 ÷ 1.4 = 5935.7 L
6. P = 2 atm.
T = 1° C (Converting Celcius into Kelvin: 273 + 1 = 274 K)
Given mass of Ne = 23g
The molar mass of Neon = 20g
Number of moles of Neon = Given mass ÷ Molar mass
23 ÷ 20 = 1.15 moles.
Substituting the values in the above-given equation:
V = 1.15 × 8.314 × 274 ÷ 2 = 1139.01 L
7. n = 11 moles.
V = 15 L.
T = 300°C ( Converting Celsius into Kelvin: 300+273 = 573 K)
Substituting values in the above given equation:
P = 11 × 573 × 8.31 ÷ 15 = 3491.8 atm
3491.8 atm into Torr = 26537 Torr
8. P = 6.5 atm.
V = 9.3 L.
n = 2.3 moles.
Substituting the values in the given equation:
T = 6.5 × 9.3 ÷ 2.3 × 8.314 = 218 Kelvin
218 Kelvin into Celsius is 55°C.
9. V₁ = 600mL.
P₁ = 700 mm
T₁ = 24° Celsius ( Converting Celsius into Kelvin: 24+273 = 297 K)
P₂ = 400 mm
T₂ = 5° Celsius ( Converting Celsius into Kelvin: 273+5 = 278K)
Substituting the values in the equation:
P₁V₁T₁ = P₂V₂T₂
V₂ = 700 × 600 × 297 ÷ 400 × 278 = 1121.7 L.
Learn more about, the ideal gas equation:
https://brainly.com/question/15379358
#SPJ1
Which two elements are found in all minerals on the chart
Answers
Oxygen and silicon are the elements found in silicon oxide compounds.
What two elements are found in silicon oxide?Silicon and oxygen, the two most usual chemical elements in the Earth's crust, combine as silicon dioxide to form the mineral silicon oxide. Quartz is the most plentiful mineral in the Earth's crust. Most of the minerals and other geological materials we see obtain from the crust, but some come from the uppermost part of the stole. In both places, oxygen and silicon are the ruling elements. Minerals can be classified by several different characteristics. The most common classification of minerals is found in chemical composition. Element is a substance that can't be broken down into simpler substances. An element is composed of atoms that have the same atomic number i.e. number of protons in its nucleus.
So we can conclude that Popular minerals are quartz, feldspar, gallium, cobalt, talc, and pyrite. Some minerals have another colored streak than their body
Learn more about minerals here: https://brainly.com/question/15844293
#SPJ1
When 0. 50 liter of a 12 m solution is diluted to 1. 0 liters, the molarity of the new solution is:.
Answers
Molarity is the concentration of a solution. It is expressed as moles of solute per liter of solution.
When 0.50 liters of a 12 M solution is diluted to 1.0 liters, the molarity of the new solution is calculated as follows:
Given, Volume of initial solution, V1 = 0.50 L
Volume of final solution, V2 = 1.0 L
Concentration of initial solution, C1 = 12 M
The amount of solute in the initial solution is given by the formula:
amount of solute = C1 * V1
So, Amount of solute in the initial solution = 12 * 0.50
= 6 moles of solute
In the final solution, the amount of solute is still 6 moles of solute.
Since the volume of the final solution has increased to 1.0 liters, the molarity of the final solution can be calculated by using the formula:
molarity = amount of solute / volume of solution
Therefore, the molarity of the final solution is:
molarity = amount of solute / volume of solution
= 6 moles / 1.0 L = 6M
Therefore, the molarity of the new solution is 6M.
To know more about Molarity visit:
brainly.com/question/2817451
#SPJ11
The solubility product constant at 25°C for AgI(s) in water has the value 8.3 × 10–17. Calculate ∆Grxn at 25°C for the process AgI(s) <--> Ag+(aq) + I– (aq) where [Ag+] = 9.1 × 10–9 and [I–] = 9.1 × 10–9. –91.7 kJ/mol +91.7 kJ/mol 0.0 kJ/mol –4.4 kJ/mol +4.4 kJ/mol
Answers
Answer:
+91.7 KJmol-1
Explanation:
Recall that ∆G= -RTlnK
Since ∆G in this case is ∆Grxn and K is the Ksp
Note that the Ksp is the solubility product (as shown by the reaction equation)
∆Grxn is the change in free energy for the reaction, in this case the ionization of the silver iodide into silver and iodide ions.
R= 8.314JK-1 and T =25°C +273 = 298 K (the centigrade temperature must be appropriately converted to its corresponding absolute absolute before proceeding with the calculation)
Hence we can substitute values accordingly;
∆Grxn = -(8.314 × 298 × ln 8.3×10^-17)
∆Grxn = +91.7 KJmol-1
Although tom was a poor student, he studied very well
Answers
True or False: Since baking soda is a powder, all the substances it breaks down into
are also powders.
Answers
Pls predict and balance the following Chemical equations 30points (5 per question)
Will mark Brainliest
1. Na2(SO4) (aq) + Ba(NO3)2(aq) -->
2. Na(NO3)(aq) + NH4Cl(aq) -->
4. ZnCl2 (aq) + K (SO4) (aq) -->
5. ZnCl2 (aq) + Na2S (aq) -->
6. Na(C2H3O2) (aq) + Ag(NO3) (aq) -->
Answers
Answer:
1. Na2SO4 + Ba(NO3)2 → NaNO3 + BaSO4
2. NaNO3 + NH4Cl → NaCl + NH4NO3
4. Sorry, I don't know
5. Refer to the attachment..
6. NaC2H3O2(aq) + AgNO3(aq) = NaNO3(aq) + AgC2H3O2
Hope it can help you and please mark me as a brainlist...\( \huge{\blue{Thank \: you}}\)

1. Na2(SO4) (aq) + Ba(NO3)2(aq) -->
Answer: NaNO3 + BaSO4 - Chemical Equation Balancer.
2. Na(NO3)(aq) + NH4Cl(aq) -->
Answer: NaNO3 + NH4Cl → NaCl + NH4NO3 - Balanced equation
4. ZnCl2 (aq) + K (SO4) (aq) -->
Answer: (This is an impossible reaction)
5. ZnCl2 (aq) + Na2S (aq) -->
Answer: no reaction occurs
6. Na(C2H3O2) (aq) + Ag(NO3) (aq) -->
Answer: NaC2H3O2(aq) + AgNO3(aq) = NaNO3(aq) + AgC2H3O2
(I’m not sure if all are right, I’m sorry but this is the best I could do. Hope it helps you.)
Consider the chemical equation.
CuCl2 + 2NaNO3 Right arrow. Cu(NO3)2 + 2NaCl
What is the percent yield of NaCl if 31.0 g of CuCl2 reacts with excess NaNO3 to produce 21.2 g of NaCl?
Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
49.7%
58.4%
63.6%
78.7%
Answers
Percent yield = 78.7% , the correct answer is D) 78.7%, which represents the percent yield of NaCl in the reaction.
To calculate the percent yield of NaCl in the given chemical equation, we need to compare the actual yield of NaCl with the theoretical yield. The theoretical yield is the amount of NaCl that would be produced if the reaction went to completion based on stoichiometry.
First, we need to determine the theoretical yield of NaCl. By examining the balanced equation, we can see that the stoichiometric ratio between CuCl2 and NaCl is 1:2. This means that for every 1 mole of CuCl2, 2 moles of NaCl are produced.
Step 1: Convert the mass of CuCl2 to moles using its molar mass.
Molar mass of CuCl2 = 63.55 g/mol (atomic mass of Cu) + 2 × 35.45 g/mol (atomic mass of Cl)
Molar mass of CuCl2 = 134.45 g/mol
Moles of CuCl2 = 31.0 g / 134.45 g/mol ≈ 0.231 mol
Step 2: Use the stoichiometry to calculate the theoretical yield of NaCl.
Since the stoichiometric ratio between CuCl2 and NaCl is 1:2, the moles of NaCl produced will be twice the moles of CuCl2.
Moles of NaCl (theoretical) = 2 × 0.231 mol = 0.462 mol
Step 3: Convert the moles of NaCl to grams using its molar mass.
Molar mass of NaCl = 22.99 g/mol (atomic mass of Na) + 35.45 g/mol (atomic mass of Cl)
Molar mass of NaCl = 58.44 g/mol
Theoretical yield of NaCl = 0.462 mol × 58.44 g/mol ≈ 26.96 g
Now, we can calculate the percent yield using the formula:
Percent yield = (Actual yield / Theoretical yield) × 100
Percent yield = (21.2 g / 26.96 g) × 100 ≈ 78.7%
Option D
For more such questions on Percent yield visit:
https://brainly.com/question/14714924
#SPJ8
The equation shows the reaction between magnesium and chlorine to produce magnesium chloride.
Mg + Cl2 → MgCl2
Magnesium chloride consists of Mg2+ and Cl− ions.
Describe, in terms of electrons, how magnesium chloride is produced from magnesium and chlorine.
Answers
Answer: Mg is a cation and Cl is an anion
Explanation: In more detail, if you look at the periodic table, the group number (or columns) of the elements, describe how many valence electrons, or how many electrons the element has available to have a complete an electron shell. Magnesium being in group 2, it has one 2 electrons that will be "donated" to an anion that it bonds with. However, Chlorine being in group 17, (look at the single digit number for double digit groups), it has 7 valence electrons. Knowing that a full electron shell is usually 8 electrons, it needs only one. Since Magnesium has 2 valence electrons to give away, two atoms of Chlorine gas will then bind to the Magnesium, where the 2 Chlorine atoms will take the electrons to fill their shells. Magnesium Chloride, in terms of charges when in ions, [Mg]2+ and 2 [Cl]-1
Magnesium chloride is formed when magnesium ion formed from the loss of two electrons and two chloride ions formed by accepting one electron each are held together by electrostatic attractive forces.
How is magnesium chloride formed from magnesium and chlorine?Magnesium chloride is an ionic compound which consists of magnesium ions and chloride ions.
A Magnesium atom forms ion by giving up two electronsTwo Chlorine atoms become ions by accepting one electron each.The oppositely-charged ions are attracted to each other.Therefore, magnesium chloride is formed when magnesium ion formed from the loss of two electrons and two chloride ions formed by accepting one electron each are held together by electrostatic attractive forces.
Learn more about ionic compound at: https://brainly.com/question/13690700
A physical property of a substance
Answers
Answer:
Hi, There!
Mass. Density. Volume. Boiling point. Melting point. Conductivity. Heat capacity.
These Are All examples Of A physical property of a substance!
xXxAnimexXx
Have a great day!
3. based on the solubility observations, which of the following pairs of cations could be distinguished by the addition of sodium chloride to the solutions? Choose.. .barium and lead barium and aluminum lead and silver iron and calcium.
Answers
The pair of cations out of which one will form a precipitate and the other will not be able to the pair that can be distinguished on the addition of sodium chloride :
1) Barium and Lead → Barium will not form a precipitate but Lead will form PbCl₂. Thus this can be distinguished.
2) Barium and Alumunium → Both will not form a precipitate.
3) Lead and Silver → Both will give precipitate so cant is distinguished.
4) Iron and Calcium → Both will not form a precipitate.
Solubility is described because the maximum amount of a substance on the way to dissolve in a given amount of solvent at a targeted temperature.
Solubility can be categorized into 3 classes. Those are soluble, partly soluble, or insoluble. A soluble solute has a solubility of extra than 1g in step with a hundred ml of solvent.
Solubility is described as the most quantity of a substance that can be absolutely dissolved in a given quantity of solvent and represents an essential idea in fields of research which include chemistry, physics, meals technological know-how, pharmaceutical, and organic sciences.
Learn more about Solubility here:-https://brainly.com/question/24057916
#SPJ4
4. Describe the arrangement of the electrons on each energy level.
Answers
Answer:
Explanation:
If the energy of an atom is increased, an electron in the atom gets excited. To go back to its ground state, the electron releases energy. The energy of the light released when an electron drops in energy level is the same as the difference in energy between the two levels.
Viewed simply, electrons are arranged in shells around an atom’s nucleus. Electrons closest to the nucleus will have the lowest energy. Electrons further away from the nucleus will have higher energy. An atom’s electron shell can accommodate 2n2 electrons (where n is the shell level).
In a more realistic model, electrons move in atomic orbitals, or subshells. There are four different orbital shapes: s, p, d, and f. Within each shell, the s subshell is at a lower energy than the p. An orbital diagram is used to determine an atom’s electron configuration.
There are guidelines for determining the electron configuration of an atom. An electron will move to the orbital with lowest energy. Each orbital can hold only one electron pair. Electrons will separate as much as possible within a shell.
In an atom, electrons are arranged in shells that surround the nucleus, with each successive shell moving further away from the nucleus.
What is energy level?In physics, an energy level is any discrete value from a set of total energy values for a subatomic particle confined by a force to a limited space or for a system of such particles, such as an atom or a nucleus.
Energy levels are fixed distances from an atom's nucleus where electrons can be found. Electrons are tiny, negatively charged particles that move around an atom's positive nucleus. The steps of a staircase represent energy levels.
Around the nucleus, electrons are arranged in different shells. Each successive shell can only hold so many electrons. First, the innermost shell is filled. This shell can only contain two electrons.
Thus, this way, the electrons are arranged.
For more details regarding energy level, visit:
https://brainly.com/question/17396431
#SPJ6
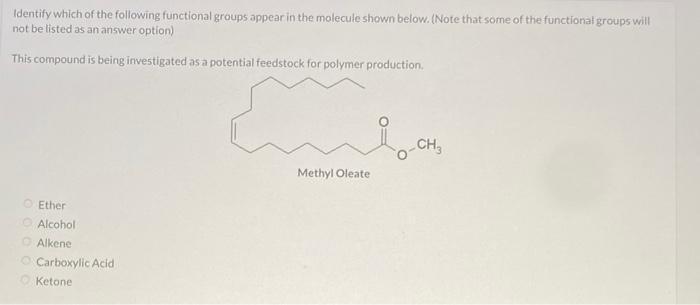
identify which of the following functional groups appear in the molecule shown below. (note that some of the functional groups will not be listed as an answer option)
Answers
The functional groups that we have in the compound are ketone and alkene. Options C and E
What is a functional group?
A functional group is a particular set of atoms in a molecule that are in charge of the molecule's distinctive chemical processes and characteristics. The behavior and functionality of a molecule in chemical processes are determined by a reactive component of the molecule.
The common atoms found in functional groups are carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus. They can be recognized by their unique atom arrangement and bonding structure inside a molecule.
Learn more about functional group:https://brainly.com/question/1356508
#SPJ4
What are some similarities and differences between group 1, 0 and 7 elements on the periodic table?
Answers
Answer:
Difference : group 1 elements are known as alkali metals, group 7 elements are known as transition metals
Similarities : Both group 1 and 7 elements become more reactive as u go down and up respectively
Which method could you use to
encourage more product, HI, to form
from the reaction below?
51.8kJ + H_{2}(g) +l 2 (g)
Answers
We can form more HI by increasing the temperature.
What is the endothermic reaction?An endothermic reaction is a chemical reaction that absorbs energy from its surroundings, typically in the form of heat. This means that the energy of the reactants is lower than the energy of the products, and the reaction requires an input of energy to proceed.
In an endothermic reaction, the temperature of the surroundings usually decreases because energy is being taken in from the environment. For example, when ice melts, the process is endothermic because the ice absorbs heat from its surroundings, causing the temperature to drop.
Learn more about endothermic reaction:https://brainly.com/question/23184814
#SPJ1
Answer:Remove HI gas
Explanation:Acellus confirmed
What does the oxidation number for elements of first transition series range between ?
Answers
The range of the of the oxidation number of the first transition series is +2 to +6.
What is transition metal?
Transition elements or transition metals are elements or metals that have partially filled d orbitals.
Examples of first transition metalsThe first main transition series begins with either;
scandium (Sc, atomic number 21)titanium (Ti, atomic number 22) chromium (Cr, atomic number 24) and ends with zinc (Zn, atomic number 30)Range of oxidation number of transition metalsscandium - oxidation number = +3titanium - oxidation number = +2, +3, and +4Chromium - oxidation number = + 6zinc - oxidation number = +2Thus, the range of the of the oxidation number of the first transition series is +2 to +6.
Learn more about oxidation number here: https://brainly.com/question/27239694
#SPJ1
To bring heavy boxes down a flight of stairs, Tyrone uses a ramp He slides the boxes down the ramp The movement transforms one type of energy into two other forms. Which of the following describes the way that most of
the energy is transformed?
potential energy into chemical energy and mechanical energy
O potential energy into light energy and mechanical energy
O potential energy into sound and chemical energy
O potential energy into heat and kinetic energy
Answers
Answer:
potential energy into heat and kinetic energy.
Explanation:
The movement transforms one type of energy into two other forms. The potential energy into heat and kinetic energy describes the way that most of the energy is transformed. Therefore, option D is correct.
What energy is an object's movement creates energy?Kinetic energy is the energy associated with an object's motion. Kinetic energy is present in a speeding bullet, a walking person, and electromagnetic radiation such as light.
The energy associated with the constant, random bouncing of atoms or molecules is another example of kinetic energy.
Thermal potential energy is potential energy at the atomic and molecular levels when particles have the ability to convert to kinetic energy. Thermal kinetic energy, on the other hand, occurs when atoms and molecules begin to move as a result of heat and temperature.
Thus, option D is correct.
To learn more about the energy, follow the link;
https://brainly.com/question/1932868
#SPJ6
Now that the chemical reaction is balanced, find the stoichiometric ratio of the reactants.

Answers
Answer: A
Explanation: A