what is one factor that adds to the greenhouse effect a decrease in the amount of dust in the atmosphere increasing amount of water on Earth's surface and increase and gases in the atmosphere that absorbs heat and increase in the amount of solar radiation that reflects into space
Answers
Human activities such as agriculture, fuel combustion, wastewater management, and industrial processes are increasing the amount of N2O in the atmosphere. Nitrous oxide is also naturally present in the atmosphere as part of the Earth's nitrogen cycle, and has a variety of natural sources
Related Questions
Who used cathode ray tubes and what discovery did this lead to?.
Answers
which product produce the two types of fermentation during production ?
Answers
Answer:
hope this helps
Explanation:
There are many types of fermentation that are distinguished by the end products formed from pyruvate or its derivatives. The two fermentations most commonly used by humans to produce commercial foods are ethanol fermentation (used in beer and bread) and lactic acid fermentation (used to flavor and preserve dairy and vegetables).
Which source of evidence did Wegener use to support his theory of continental drift?
fossils found on Earth
magnetic fields of Earth
satellite mapping of the tropical islands
glaciers found near the poles
Answers
Answer:
A: fossils found on Earth
Explanation:
TOOK TEST
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
Which of the following will be formed when lead storage battery is charged
Answers
Answer:
During the charging of the lead storage battery, the reactions get reversed and the cathode becomes anode and anode becomes cathode.
The reactions during charging that occurs at anode are:
PbSO4(s) +2e → PB(s) + SO4 2-(aq)
The reactions during charging that occurs at cathode are:
PbO2(S) + 2H2O → PbSO2(s) + SO4 2-
The overall reaction of charging can be written as:
2PBSO4(s) + 2H2O(l) → PB(s) + PBO2(s) +2H2SO4(aq)
During the charging of the lead storage battery, concentration of hydrochloric acid (H2SO4) increases because it is regenerated.
Explanation:
The lead storage batteries are used in automobiles such as in cars, trucks, buses, etc. In lead storage batteries, the anode is a group of lead plates and cathode is considered as lead dioxide. In lead storage cells the electrolyte is sulfuric acid.
Can someone please answer this.

Answers
determine how many millilitres of a 4.25 M HCl solution are needed to react completely with 8.75 g CaCO3?
2HCl + CaCO3 --> CaCl2 + CO2 + H2O
Answers
Answer:
41 mL
Explanation:
Given data:
Milliliter of HCl required = ?
Molarity of HCl solution = 4.25 M
Mass of CaCO₃ = 8.75 g
Solution:
Chemical equation:
2HCl + CaCO₃ → CaCl₂ + CO₂ + H₂O
Number of moles of CaCO₃:
Number of moles = mass/molar mass
Number of moles = 8.75 g / 100.1 g/mol
Number of moles = 0.087 g /mol
Now we will compare the moles of CaCO₃ with HCl.
CaCO₃ : HCl
1 : 2
0.087 : 2/1×0.087 = 0.174 mol
Volume of HCl:
Molarity = number of moles / volume in L
4.25 M = 0.174 mol / volume in L
Volume in L = 0.174 mol /4.25 M
Volume in L = 0.041 L
Volume in mL:
0.041 L×1000 mL/ 1L
41 mL
For alkyl halides used in SN1 and SN2 mechanisms, rank the leaving groups in order of reaction rate. You are currently in a ranking module. Turn off browse mode or quick nav, Tab to move, Space or Enter to pick up, Tab to move items between bins, Arrow Keys to change the order of items, Space or Enter to drop.
Answers
Answer:
Iodide> Bromide > chloride > flouride
Explanation:
During a nucleophilic substitution reaction, a nucleophilie replaces another in a molecule.
This process may occur via an ionic mechanism (SN1) or via a concerted mechanism (SN2).
In either case, the ease of departure of the leaving group is determined by the nature of the C-X bond. The stronger the C-X bond, the worse the leaving group will be in nucleophilic substitution. The order of strength of C-X bond is F>Cl>Br>I.
Hence, iodine displays the weakest C-X bond strength and it is thus, a very good leaving group in nucleophillic substitution while fluorine displays a very high C-X bond strength hence it is a bad leaving group in nucleophilic substitution.
Therefore, the ease of the use of halide ions as leaving groups follows the trend; Iodide> Bromide > chloride > flouride
Which of the following is an example of a Mechanical Wave.
Sound Waves
O X-Rays
O Light Waves
O Ultraviolet Light
Answers
Answer:
sound waves
Explanation:
hope this helps
Define biotechnology. } List two advantages in the use of biotechnology
Answers
Advantages of biotechnology:
Improvement of plants and animal breeds to give a high yield of their products.
Pests and pathogen control in agriculture which will reduce the loss of yield in food crops.
Synthesis of biocatalyst which can be used for enhancing the reactions which can be carried out in vitro or laboratory conditions.
Sewage treatment or water recycling can be done with the help of transgenic microbes which have better efficiency and speed.
Biotechnology is the use of living organisms or other biological systems in the manufacture of drugs or other products or for environmental management, as in waste recycling: includes the use of bioreactors in manufacturing, microorganisms to degrade oil slicks or organic waste, genetically engineered bacteria to produce human hormones, and monoclonal antibodies to identify antigens.
Biotech offers the possibility of improving human health, the environment, and agriculture while creating more sustainable modes of production.
A chemist records the amount of liquid dispensed by a machine and obtains the following values: 283.2 mL, 284.1 mL, 283.9 mL, 284.0 mL, 284.1 mL. The machine is supposed to dispense 296 mL of liquid. Which of the following describes the accuracy and precision of the machine?
precise but not accurate
accurate but not precise
neither accurate nor precise
accurate and precise
Answers
The machine is accurate and precise
Hence , option D is correct one .
Accuracy is the capacity of an instrument to measure the precise value. In other words, it refers to how closely the measured value resembles a reference or genuine value. Small readings can be taken to increase accuracy. The calculation's inaccuracy is decreased by the little reading.
The precision of a substance is defined as the degree to which two or more measurements agree with one another. It is incredibly precise but not always accurate to measure something if you weigh it five times and get 3.2 kg each time. Accuracy is not necessary for precision.
learn about precise ,
https://brainly.com/question/15926220
#SPJ1
A student wishes to prepare 25mL of 0.1M NaOH from 6M NaOH.
How many mL of 6M NaOH need to be placed in a 25.00mL volumetric flask for dilution to generate
25mL of a 0.1M solution?
Answers
Answer:
Na(OH)4
Explanation:
Look at the charges and add them up
Na(OH) Na(OH)
1. If a reaction starts with 5 atoms of Hydrogen, how many will there be
after they react with 10 atoms of Oxygen?
Answers
Answer:
When a chemical reaction starts with 5 atoms of hydrogen, and the reaction goes into completion, it must give us 5 atoms of hydrogen.
This is in compliance with the law of conservation of mass which states that "in a chemical reaction, matter is neither created nor destroyed but converted from one form to another".
In chemical reactions, bonds are broken and new compounds are formed in the process. The same number of species at the start and end of the reaction must still be the same.
Explanation:
What are some potential real-world applications for renewable energy sources such as solar power and wind power?
Answers
The some of the potential in the real world applications for the renewable energy sources such as the solar power and the wind power are electricity generation, the water heating and cooling, and the transportation.
Renewable energy defined as the energy produced from the sources like the sun and the wind energy which are the naturally replenished and which do not run out.
The Renewable energy which can be used for the electricity generation, and the water heating and the cooling, and the transportation. The most sustainable sources of the energy are the renewable bioenergy. The Renewable sources of the, like the wind and the solar, it will emit the little to no the greenhouse gases.
To learn more about renewable energy here
https://brainly.com/question/18004988
#SPJ1
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
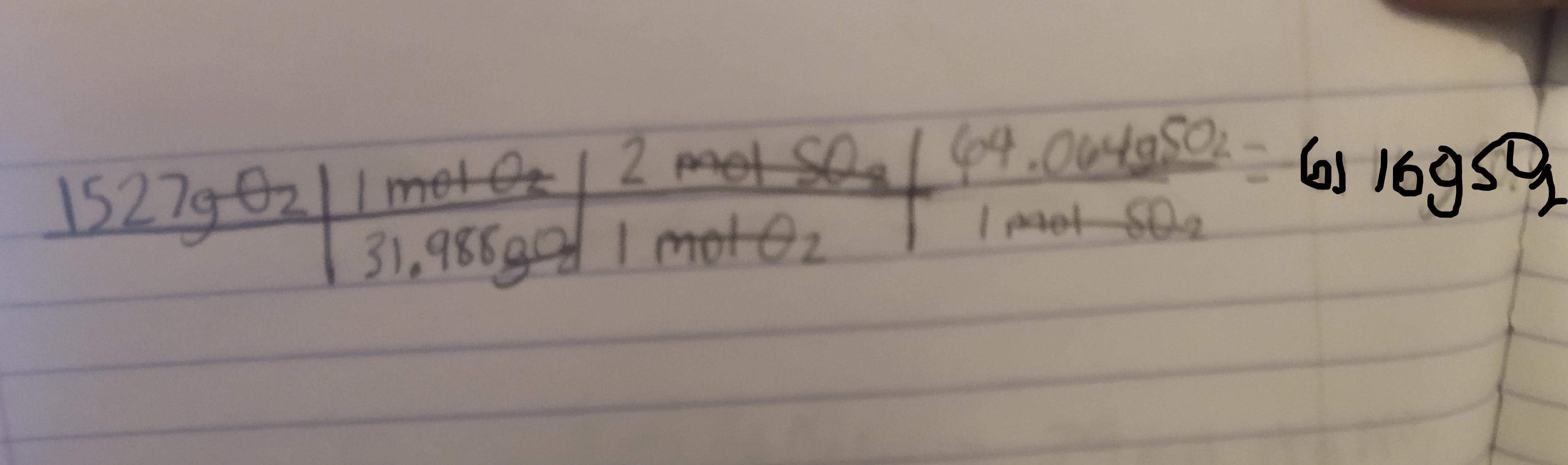
In scenario five, you have the same number of hydrogen and oxygen molecules to start off. Howmany water molecules are made? What is the limiting reactant? What is the excess reactant?

Answers
The balanced equation for the production of water is:
2 H2 + O2 -> 2 H2O
In the question we are given 2 moles of H2, and 2 moles of O2, but from what we have seem, we will only need 1 mol of O2 in order to form 2 moles of H2O, therefore the limiting reactant will be H2 and the excess reactant will be O2
What mass of water can be heated from 15.0° C to 43.1° C by the addition of 1282 J?
Answers
The mass of water that can be heated from 15.0° C to 43.1° C by the addition of 1282J is 10.9grams.
How to calculate mass?The mass of water can be calculated using the following expression;
Q = mc∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of substancec = specific heat capacity∆T = change in temperatureAccording to this question, a sample of water can be heated from 15.0° C to 43.1° C by the addition of 1282J. The mass of the substance can be calculated as follows;
1282 = m × 4.184 × (43.1 - 15)
1282 = 117.57m
m = 10.9grams
Learn more about mass at: https://brainly.com/question/31584398
#SPJ1
How do the chemical reactions in this lab activity compare to nuclear reactions, such as fission and fusion?
Answers
The chemical reaction has been the low energy reaction containing electrons rearrangement, while nuclear reactions have been the higher energy reactions with change in nuclei.
What are nuclear reactions ?The term nuclear reactions is defined as when there has been the including the change in the nuclei of the atom. However, the reaction has been known as the chemical reaction when the change has been processed in the electrons with the rearrangement.
The nuclear reactions have made up of a more amount of energy to be liberated, while the amount of energy included in the chemical reaction has been smaller.
Thus, the chemical reaction has been the low energy reaction including electrons rearrangement, while nuclear reactions have been the high energy reactions with change in nuclei.
For more information about the nuclear reactions, refer to the link:
https://brainly.com/question/16526663
#SPJ2
hello I need help with this question the test tube is referring to is wooden splint test

Answers
Answer and explanation
To collect a gas, a beaker is filled with water and then turned upside down in a bucket of water, this is done to make sure that there is no gas or air in the beaker, so that the correct amount of gas produced is recorded.
The water level goes down during an experiment because the gas produced replaces the water, the gas bubbles force some of the water out of the beaker.
The purpose of water is to fill the container so that the gas that will be collected is pure and not mixed with air, so water acts as a barrier, to prevent air into the beaker.
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
How does the automatic hammer convert gravitational potential energy into kinetic energy?
Answers
Answer:
Exolaiend in explanation section
Explanation:
First of all, the automatic hammer is used to drive nails into tight spaces where where we can't get a sufficient striking force if we are to use a normal regular hammer in driving the nail.
So the nail to be driven is lifted out of rest(it's position). The energy here is gravitational potential energy.
Now, when it is driven into the tight spaces, the gravitational energy would be converted to kinetic energy due to the motion and speed involved.
please help fast :/
how many moles are present in 60 grams of hydrochloric acid , HCI ?
a : 1.40 moles
b : 36.45 moles
c : 1.65 moles
d : 60 moles
Answers
Answer: C- 1.65 moles
Explanation:
HCl = 1 + 35.45 = 36.45 g/mol
Check all that apply...helppppp

Answers
Answer:
dfgh
Explanation:
What makes a balloon electrically charged?
Adding more air to the balloon
Placing the balloon against a wall
Rubbing the balloon against your hair
Running water over the balloon
Answers
Explanation:
rubbing it against your hair
Answer:
C
Explanation:
Hope this helps and have a great day.
15.0 L of an ideal gas at 298 K and 3.36 bar are heated to 350 K with a new pressure of 4.40 atm. What is the new volume in litres?
Answers
Answer:
13.3 L
Explanation:
Step 1: Given data
Initial pressure (P₁): 3.36 barInitial volume (V₁): 15.0 LInitial temperature (T₁): 298 KFinal pressure (P₂): 4.40 atmFinal volume (V₂): ?Final temperature (T₂): 350 KStep 2: Convert P₁ to atm
We will use the conversion factor 1 atm = 1.01325 bar.
3.36 bar × (1 atm / 1.01325 bar) = 3.32 atm
Step 3: Calculate V₂
We will use the combined gas law.
P₁ × V₁/T₁ = P₂ × V₂/T₂
V₂ = P₁ × V₁ × T₂/T₁ × P₂
V₂ = 3.32 atm × 15.0 L × 350 K/298 K × 4.40 atm
V₂ = 13.3 L
24) Which ionization process requires the most energy?
A) W(g) → W+(g) + e-
B)W+ (g)→ W2+ (g)+e-
C)W2+ (g)→ W3+ (g)+e-
D)W3+ (g)→ W4+ (g)+e-
E) All the above reactions require the same amount of energy.
Answers
The ionization process that requires the most energy is; W³⁺(g) → W⁴⁺(g) + e⁻. Option D is correct.
Ionization is the process of converting an atom or molecule into an ion by adding or removing electrons. An ion is an atom or molecule that has a net electrical charge because it has either gained or lost one or more electrons.
Therefore, of the given ionization processes, the ionization process that requires the most energy is; W³⁺(g) → W⁴⁺(g) + e⁻
This is because we are removing an electron from a positively charged ion (W³⁺), which requires more energy than removing an electron from a neutral atom or a singly charged ion.
Option A involves removing only one electron from a neutral atom, while options B and C involve removing an electron from a singly charged ion. Therefore, they require less energy than option D.
Option E is not correct because the ionization energies of different processes are not necessarily the same.
Hence, D. is the correct option.
To know more about ionization here
https://brainly.com/question/16243729
#SPJ1
An energy of 4.50x10^-19 J/photon was released when an electron drops to a lower energy state, what is the wavelength of the photon? What color does this energy correspond to?
Answers
4.41 × 10⁻⁴⁵m is the wavelength of the photon and the energy correspond to red color.
What do you mean by the wavelength ?The term wavelength is defined as the distance between two identical points that are adjacent crests and troughs.
The SI unit of wavelength is metre mostly represented as m.
The wavelength is mostly represented by λ is the Greek letter lambda.
Given:
E = 4.50x10⁻¹⁹ J/photon
h = Planck constant = 6.626 × 10⁻³⁴
ν = ?
E = hν
ν = E/h
By substituting the values in above question and we get,
= 4.50x10^-19 / 6.626 × 10⁻³⁴
= 0.679 × 10⁻¹⁵
c = 3 × 10⁸
E = hc/λ
λ = hc/E
By substituting the values in above question and we get,
λ = 6.626 × 10⁻³⁴ × 3 × 10⁸ / 4.50x10⁻¹⁹
λ = 4.41 × 10⁻⁴⁵m
Thus, the wavelength of the photon is 4.41 × 10⁻⁴⁵m and color does this energy correspond to red.
To learn more about the wavelength, follow the link;
https://brainly.com/question/13533093
#SPJ9
Identify the products formed in this Brønsted-Lowry reaction.
HPO2−4+NO−2↽−−⇀acid+base
Answers
Answer: The products formed in this Bronsted-Lowry reaction are \(HNO_{2}\) and \(PO^{2-}_{4}\).
Explanation:
According to Bronsted-Lowry, acids are the species which donate hydrogen ions to another specie in a chemical reaction.
Bases are the species which accept a hydrogen ion upon chemical reaction.
For example, \(HPO^{2-}_{4} + NO^{-}_{2} \rightleftharpoons HNO_{2} + PO^{2-}_{4}\)
Here, the products formed in this Bronsted-Lowry reaction are \(HNO_{2}\) and \(PO^{2-}_{4}\).
Thus, we can conclude that the products formed in this Bronsted-Lowry reaction are \(HNO_{2}\) and \(PO^{2-}_{4}\).
What is the chemical formula for sulfuric acid?
Answers
Which of the following is a possible
way to describe the SO3 component in
the reaction below?
Sa(s) + 120₂(g) → 8SO3(g)
A. 8 atoms SO3
B. 8 molecules SO3
C. 80.07g SO3
D. 32 LSO3
Answers
The correct answer is B. 8 molecules \(SO_3\). Option B
In the given reaction:
S(s) + \(O_2\)(g) → \(SO_3\)(g)
The stoichiometric coefficient in front of the \(SO_3\)molecule is 8, which indicates that 8 molecules of \(SO_3\)are formed as a product. This coefficient represents the ratio of the number of molecules involved in the reaction.
Option A (8 atoms \(SO_3\)) is incorrect because it only mentions the number of atoms, not molecules. The stoichiometric coefficient does not represent the number of atoms, but rather the number of molecules.
Option C (80.07g \(SO_3\)) is incorrect because it mentions a specific mass. The stoichiometric coefficient does not directly represent the mass of the substance, but rather the relative amount of molecules involved in the reaction.
Option D (32 \(SO_3\)) is incorrect because it mentions a specific volume. The stoichiometric coefficient does not directly represent the volume of the substance, but rather the relative amount of molecules involved in the reaction.
Therefore, the correct way to describe the \(SO_3\)component in the reaction is option B: 8 molecules \(SO_3\). This represents the ratio of the number of molecules of \(SO_3\)that are produced in the reaction.
Option B
For more wsuch question on molecules visit:
https://brainly.com/question/475709
#SPJ8