what is density?????? any help anyone
Answers
Answer:
Density, mass of a unit volume of a material substance
Explanation:
Answer:
Hello!
Explanation:
Density: The degree of compactness of a substance.
happy to help!
Related Questions
1. How is extinction different from mass extinction?
Answers
Answer:
Extinction is the elimination of one species whereas mass extinction results in multiple animals to become extinct.
3. SE7.12D The cell organelle responsible for providing energy for cell processes in plant
and animal cells is the -
Answers
Explain why most volcanoes occur at plate boundaries and which two types of boundaries are most common.
Please explain why your answer is correct
Answers
Most volcanoes occur at plate boundaries because the tectonic plates are moving away from one another and the Earth's crust is pulled apart to create a new pathway for rising hot magma to flow on to the surface while the two types of boundaries which are most common are:
Divergent plate boundaries.Convergent plate boundaries.What is a Volcano?This is referred to as a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
It occurs at the plate boundaries because Earth's crust is pulled apart to create a new pathway for rising hot magma to flow on to the surface and the types which are most common are divergent plate boundaries which includes two tectonic plates that are moving away from each other and convergent plate boundaries which involves two tectonic plates moving toward each other.
Read more about Volcano here https://brainly.com/question/25121802
#SPJ1
what would be the water potential of pure water at atmospheric pressure?
Answers
Answer:Zero is the value of water potential of pure water at atmospheric pressure
Explanation:
i hopes it helps
1) Do the following processes produce an increase or decrease in entropy? Justify A) H2O(1) - H2O(g) B) N(g) + O(g) - NO(g) C) N2(g) + 3H2(g) - 2NH3(g) D) C&His(g) + 2502(g) 16CO2(g) + 18H20(g) E) CaO(s) + CO2(g) → CaCO3(s) F) MgCl2(s) + H2O(1) ► Mg(s) + 2HCl()
Answers
Three reaction result in Increase in entropy and three result in decrease in entropy.
How we determine entropy for different reactions?A) H2O(1) - H2O(g): Increase in entropy.
This is because when water goes from a liquid to a gas, the number of available microstates increases, which leads to an increase in entropy.
B) N(g) + O(g) - NO(g): Increase in entropy.
This is because the formation of NO from N and O involves an increase in the number of particles, which leads to an increase in the number of microstates and hence an increase in entropy.
C) N2(g) + 3H2(g) - 2NH3(g): Decrease in entropy.
This is because the reactants have higher entropy than the products. The formation of NH3 involves a decrease in the number of particles and an increase in order, which leads to a decrease in entropy.
D) C&His(g) + 2502(g) 16CO2(g) + 18H20(g): Decrease in entropy.
This is because the reactants have higher entropy than the products. The combustion of carbon and hydrogen with oxygen involves a decrease in the number of particles and an increase in order, which leads to a decrease in entropy.
E) CaO(s) + CO2(g) → CaCO3(s): Decrease in entropy.
This is because the reactants have higher entropy than the products. The formation of CaCO3 from CaO and CO2 involves a decrease in the number of particles and an increase in order, which leads to a decrease in entropy.
F) MgCl2(s) + H2O(1) ► Mg(s) + 2HCl(): Increase in entropy.
This is because the reaction involves an increase in the number of particles and an increase in the number of microstates, which leads to an increase in entropy.
Have more Question about entropy Learn here
brainly.com/question/13135498
#SPJ11
What is crystallization in the rock cycle?
Answers
Answer:
Crystallization. Magma cools either underground or on the surface and hardens into an igneous rock. As the magma cools, different crystals form at different temperatures, undergoing crystallization. For example, the mineral olivine crystallizes out of magma at much higher temperatures than quartz.
1 point
Using the equation 4HCl(aq) + O2(g) + 2Cl2(g) + 2H₂O(g) +20kJ, if [HCI]=0.302,[0₂]=0.109,[Cl₂]=0.883,[H₂O]=0.166, find Keq.
23.7
7.5
Answers
Answer:
Therefore, the equilibrium constant (K) for the given reaction is 23.7.
Explanation:
To calculate the equilibrium constant (K), we need to use the law of mass action. The law of mass action states that the product of the concentrations of the products raised to their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients is equal to the equilibrium constant.
The balanced chemical equation is:
4HCl(aq) + O2(g) + 2Cl2(g) + 2H₂O(g) ⇌ 4ClH0.5(aq)
The stoichiometric coefficients indicate that the reaction involves a one-to-one ratio of reactants to products. Therefore, we can write:
K = [ClH0.5]^4 / [HCl]^4 [O2] [Cl2]^2 [H2O]^2
Substituting the given concentrations into the expression, we get:
K = [(0.883/2)^4] / [(0.302)^4 (0.109) (0.883)^2 (0.166)^2]
Simplifying the expression and calculating, we get:
K = 23.7
Therefore, the equilibrium constant (K) for the given reaction is 23.7.
IWhich of the following solutions would be most likely to have the highest water concentration?
Multiple Choice hypertonic solution isotonic solution hypotonic solution water concentration and tonicity of a solution cannot be compared
Answers
"Hypotonic solution," refers to a solution with the highest water concentration due to its lower solute concentration compared to the other options, leading to water influx into cells.
A hypotonic solution would be most likely to have the highest water concentration. In a hypotonic solution, the solute concentration is lower than that inside the cell or compared to another solution. As a result, water tends to move into the cell or the solution to equalize the concentration.
When a cell is placed in a hypotonic solution, water molecules will move into the cell through the process of osmosis. This influx of water increases the water concentration inside the cell, leading to cell swelling or even bursting in extreme cases.
Compared to hypertonic and isotonic solutions, a hypotonic solution has a lower solute concentration, allowing for a higher concentration of water molecules. This results in a higher water concentration in the solution. It's important to note that the concept of tonicity is related to the relative solute concentrations between two solutions and their effect on cell osmosis. In this case, a hypotonic solution is characterized by a higher water concentration compared to the other options.
learn more about Hypotonic solution here:
https://brainly.com/question/28020628
#SPJ11
A sample of ideal gas at room temperature occupies a volume of 28.0 L at a pressure of 882 torr. If the pressurechanges to 4410 torr , with no change in the temperature or moles of gas, what is the new volume, V?Express your answer with the appropriate units.
Answers
To solve this problem, we could use the Boyle's law since there's a sample of ideal gas and temperature is constant.
Boyle's law tells us that:
Replacing the values of the problem:
The new volume V equals 5.6L.


What happens when potassium metal react with nitric acid
Answers
Answer:
Astamañana vaya buenas noches
Describe the difference between an element and a compound. Give an example of each.
Answers
The difference between an element and a compound is an element is a substance that cannot be divided into simpler forms by chemical reactions. Compounds are made up of two or more different elements combined in fixed proportions.
Elements are substances that cannot be divided into simpler forms by chemical reactions. They are chemically pure and consist of atoms that have the same number of protons and electrons. The properties of elements vary depending on their atomic structure, and they are organized in the periodic table.
Compounds, on the other hand, are made up of two or more different elements combined in fixed proportions. They can be broken down into simpler substances through chemical reactions.
Elements and compounds can be differentiated by their chemical formulas. Elements are represented by a symbol, such as H for hydrogen, while compounds are represented by a combination of symbols, such as H2O for water. Elements are also classified into groups based on their physical and chemical properties.
Examples:
Example of Element: Carbon
Carbon is a chemical element with the symbol C and atomic number 6. It is a non-metallic element with a wide range of applications in various industries. Carbon exists in different forms, including graphite, diamond, and fullerene.
Example of Compound: Water
Water is a compound made up of two hydrogen atoms and one oxygen atom, represented by the chemical formula H2O. It is an essential substance for life and is used for a wide range of purposes, including drinking, cleaning, and industrial processes.
Learn more about an element and a compound from the given link-
https://brainly.com/question/33318982
#SPJ11
What is Delta. Gsystem for the system that is described by the following data? Delta. Hsystem = –232 kJ, T = 293 K, Delta. Ssystem = 195 J/K Delta. Gsystem = Delta. Hsystem – TDelta. Ssystem –289 kJ –175 kJ 256 kJ 56,903 kJ.
Answers
Delta Gsystem is -289.04 kJ for the system described by the given data. In thermodynamics, the symbol "G" stands for the Gibbs free energy of a system.
The answer to the question can be derived as follows:
Given data are;
Delta Hsystem = -232 kJ,T = 293 K,
Delta Ssystem = 195 J/K
Delta Gsystem = Delta Hsystem - TDelta Ssystem
We can now substitute these values into the equation for Delta Gsystem as follows;
Delta Gsystem = (-232 kJ) - (293 K × 195 J/K)
Delta Gsystem = (-232000 J) - (57035 J) = -289035 J
Convert this to kilojoules;
Delta Gsystem = -289.04 kJ (Answer)
Delta Gsystem is -289.04 kJ for the system described by the given data.
In thermodynamics, the symbol "G" stands for the Gibbs free energy of a system. Gibbs free energy or Gibbs energy is a measure of the maximum amount of work that can be performed by a thermodynamic system at a constant temperature and pressure (isothermal, isobaric).When a reaction occurs spontaneously, the Gibbs free energy of the system decreases and the reaction is exergonic (releases energy). When the Gibbs free energy of the system is positive, the reaction is endergonic (absorbs energy) and does not occur spontaneously.
Learn more about reaction :
https://brainly.com/question/30464598
#SPJ11
The question is on 9.) on the page I need help with

Answers
1.2x10^2 g of AlCl3 will be produced.
1st) It necessary to calculate the grams of chlorine (Cl2) and aluminum chloride (AlCl3) that are related on the given equation. Here we need to use the molar mass of each compound:
- Cl2 molar mass: 70.9 g/mol
- AlCl3 molar mass: 133 g/mol
The equation shows that with 3 moles of Cl2 we can produce 2 moles of AlCl3. With a mathematical Rule of Three and the molar mass of each compound we can calculate the grams that are needed by formula:
- Chlorine:
\(\begin{gathered} 1molCl_2-70.9g \\ 3molCl_2-x=\frac{3molCl_2\cdot70.9g}{1molCl_2} \\ \\ x=212.7g \end{gathered}\)- Aluminum chloride:
\(\begin{gathered} 1molAlCl_3-133g \\ 2molAlCl_3-x=\frac{2molAlCl_3\cdot133g}{1molAlCl_3} \\ \\ x=266g \end{gathered}\)2nd) We can calculate the grams of AlCl3 that will be produced from 92g of Cl2:
\(\begin{gathered} 212.7gCl_2-266gAlCl_3 \\ 92gCl_2-x=\frac{92gCl_2\cdot266gAlCl_3}{212.7gCl_2} \\ \\ x=1.2\cdot10^2gAlCl_3 \end{gathered}\)So, 1.2x10^2 g of AlCl3 will be produced.
guía resuelta fase 2 semana 3 ciencias 9° grado
Answers
Answer:
hi im andre and im 14
Explanation:
Nickel has a cubic unit cell. The edge of the unit cell is 3.524
x 10^(-8)cm. Determine the atomic radius of Nickel.
Answers
The approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
In a cubic unit cell, the body diagonal length (diagonal that passes through the center of the unit cell) is equal to four times the atomic radius (4r). We can use this relationship to find the atomic radius of nickel.
Given: Edge length of the unit cell (a) = 3.524 × 10^(-8) cm
The body diagonal length is given by:
Diagonal length (d) = a√3
Substituting the given values:
d = (3.524 × 10^(-8) cm) × √3
Now, we can calculate the atomic radius (r) by dividing the diagonal length by 4:
r = d / 4
Performing the calculations:
r = [(3.524 × 10^(-8) cm) × √3] / 4
r ≈ (3.524 × 10^(-8) cm) × (1.732 / 4)
r ≈ 1.532 × 10^(-8) cm
Therefore, the approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
Know more about atomic radius here
https://brainly.com/question/18095927#
#SPJ11
how do we determine the number of neutrons an element has
Answers
Answer:
To determine the amount of neutrons an element has you would subtract the number of protons/atomic number from the atomic mass.
Explanation:
The catalytic converter of a secondary air injection system converts the hydrocarbons and carbon monoxide to _______ and _______.
Answers
The catalytic converter of a secondary air injection system converts the hydrocarbons and carbon monoxide to carbon dioxide (CO2) and water (H2O). It is a fundamental part of a vehicle.
Catalytic converter and air injection systemsA catalytic converter refers to the device observed in a car and/or vehicle, which is used to remove different contaminant gases.
In general, the catalytic converter is placed close to the car engine and reduces the release of hydrocarbons.
In general, the air injection system is connected to the catalytic converter in order to provide useful oxygen required for oxidation reactions.
Learn more about catalytic converters here:
https://brainly.com/question/9106255
A chemist must prepare of aqueous copper(II) sulfate working solution. He'll do this by pouring out some aqueous copper(II) sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the copper(II) sulfate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.
Answers
Answer:
380 mL
Explanation:
A chemist must prepare 925 mL of 325 mM aqueous copper(II) sulfate working solution. He'll do this by pouring out some 0.792 M aqueous copper(II) sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the copper(II) sulfate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.
Step 1: Given data
Initial concentration (C₁): 0.792 MInitial volume (V₁): ?Final concentration (C₂): 325 mMFinal volume (V₂): 925 mLStep 2: Convert "C₂" to M
We will use the conversion factor 1 M = 1000 mM.
325 mM × (1 M/1000 mM) = 0.325 M
Step 3: Calculate the inital volume
We will use the dilution rule.
C₁ × V₁ = C₂ × V₂
V₁ = C₂ × V₂/C₁
V₁ = 0.325 M × 925 mL/0.792 M
V₁ = 380 mL
3) the following reaction is an example of a reaction. * al(s) h2so4(aq)->al2(so4)3(aq) ?h2
Answers
Redox (Oxidation-Reduction) (Oxidation-Reduction) Al2(SO4)3 + H2 from the reaction Al + H2SO4 may be a redox reaction.
Short answer: a chemical reaction?A chemical reaction is a process in which one or more substances, also known as reactants, are changed into one or more distinct compounds, known as products. Either chemical elements or chemical compounds make up substances.A chemical reaction is a process that results in the chemical conversion of one group of chemical components into another.Redox (Oxidation-Reduction) (Oxidation-Reduction) Al + H2SO4 = Al2(SO4)3 + H2 could be a redox process. A chemical reaction called an oxidation-reduction (redox) reaction includes the exchange of electrons between two substances.To learn more about Chemical reaction refer to:
https://brainly.com/question/11231920
#SPJ4
How many atoms of Ca are present in 80.156 grams of Ca?
1.2044 × 10^24
2.4088 × 10^24
4.8270 × 10^25
1.9346 × 10^27
Answers
The number of atoms present in 80.156 grams of Ca is 1.204×10²⁴ atoms
Avogadro's hypothesisFrom Avogadro's hypothesis,
1 mole of Ca = 6.02×10²³ atoms
But
1 mole of Ca = 40.078 g
Thus,
40.078 g of Ca = 6.02×10²³ atoms
How to determine the atoms in 80.156 g of Ca40.078 g of Ca = 6.02×10²³ atoms
Therefore,
80.156 g of Ca = (80.156 × 6.02×10²³) / 40.078
80.156 g of Ca = 1.204×10²⁴ atoms
Thus, 1.204×10²⁴ atoms is present in 80.156 g of Ca.
Learn more about Avogadro's number:
https://brainly.com/question/26141731
Answer:
A. 1.204e24 or 1.2044*10^24
Explanation:
I took the test
Greta passed a comb through her hair several times. She then placed the comb next to a stream of water flowing from a faucet. Greta observed the stream of water bend toward the comb. How did passing a comb through her hair allow the comb to bend the stream of water?.
Answers
Passing a comb through her hair allows the comb to bend the stream of water is option B. by giving the comb an electrical charge.
The comb draws the movement of water in an equal way. The price of the comb attracts the molecules of water within the flow. due to the fact the molecules inside the circulation may be moved without difficulty, the circulate bends toward the comb. because water molecules are polarized molecules, the effect is stronger than with dust.
While a comb is run thru your hair charges pass between your hair and the brush, so the comb turns into charged both definitely or negatively, and the hair is oppositely electrical charge.
The brush, blanketed in negatively charged electrons, becomes negatively charged as nicely, and your hair is left with a high-quality fee. This “separation of fee” is the purpose of the collection of effects we call static strength. If gadgets have specific prices, they appeal to (or pull towards) every other.
Learn more about electrical charge here:-https://brainly.com/question/2373424
#SPJ4
Disclaimer:- your question is incomplete, please see below for the complete question.
Greta passed a comb through her hair several times. She then placed the comb next to a stream of water flowing from a faucet. Greta observed the stream of water bend toward the comb. How did passing a comb through her hair allow the comb to bend the stream of water?
A. by making the comb more absorbent.
B. by giving the comb an electrical charge.
C. by increasing the thermal energy of the comb.
D. by breaking some of the chemical bombs in the comb.
(Help ASAP) in the summer many people in Argentina which is located in the southern hemisphere enjoy going to the beach which diagram to the right shows the position of earth when it is summer in Argentina?
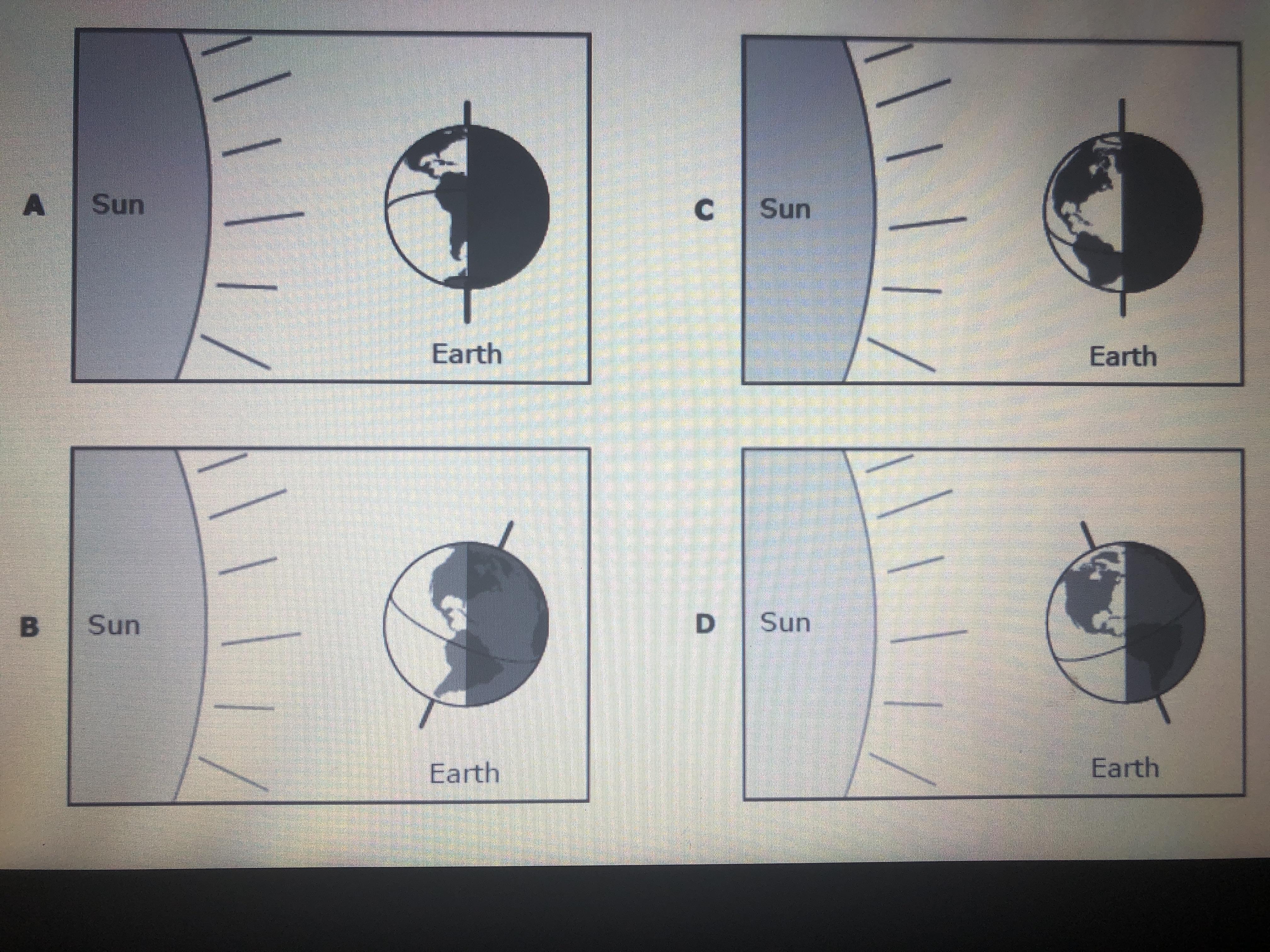
Answers
Answer:
I think it's B
Explanation:
A and C are out of the equation because Earth's axis isn't tilted on them and has the entirety of Argentina covered in shadow. The only answer left would be B.
How much water must be added to 516 mL of 0.191 M HCl to produce a 0.133 M solution? (Assume that the volumes are additive)
Answers
Answer:
225 mL of water must be added.
Explanation:
First we calculate how many HCl moles are there in 516 mL of a 0.191 M solution:
516 mL * 0.191 M = 98.556 mmol HClNow we use that number of moles (that remain constant during the dilution process) to calculate the final volume of the 0.133 M solution:
98.556 mmol / 0.133 M = 741 mLWe can calculate the volume of water required from the volume difference:
741 mL - 516 mL = 225 mLHow much of nacl is in 1.67 l of 0.400 m
nacl?
answer in units of mol.
Answers
Answer:
.668 mole
Explanation:
1.67 * .4 = .668 mole
what is chemistry ? Explain its types.
Answers
Answer:
Fundamentally, chemistry is the study of matter and change. The way that chemists study matter and change and the types of systems that are studied varies dramatically. Traditionally, chemistry has been broken into five main subdisciplines: Organic, Analytical, Physical, Inorganic and Biochemistry.
Explanation:
hope this helps
What is an ion? An atom that has more or fewer protons than it "should" An atom that has more or fewer neutrons than it "should" An atom that has more or fewer electrons than it "should" Question 5 refers to a mineral's intensity of reflected light. luster cleavage streak tenacity is defined as a mineral's resistance to scratching. cleavage streak hardness fracture Question 7 5 pts The are the mineral class that accounts for more than 90% of the Earth's crust. sulfates silicates carbonates native elements A rock can be composed of almost entirely one mineral. True False Question 9 All minerals have cleavage. True False Color is a reliable technique for the identification of minerals. True False
Answers
True or false: A rock can be composed almost entirely of one mineral.
True. All minerals do not have cleavage.
True or false: Color is a reliable technique for identifying minerals.
False.
What is an ion?
An ion is an atom that has more or fewer electrons than it "should." An atom may gain or lose electrons to become an ion, resulting in a negative or positive charge. A mineral's intensity of reflected light is referred to as luster.
The resistance to scratching is known as hardness. Cleavage is the tendency of minerals to break along planes of weakness. Tenacity refers to the minerals' resistance to deformation, bending, or breaking. The mineral class that accounts for more than 90% of the Earth's crust is silicates.
True or false: A rock can be composed almost entirely of one mineral.
True. All minerals do not have cleavage.
True or false: Color is a reliable technique for identifying minerals.
False.
To know more about composed visit:
https://brainly.com/question/14488122
#SPJ11
An ion is an atom or molecule that has lost or gained electrons, resulting in a net electric charge. The mineral class accounting for more than 90% of Earth's crust are silicates. Not all minerals have cleavage and color isn't reliable for identifying minerals.
Explanation:An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons. The process producing ions is called ionization.
'Luster' refers to a mineral's intensity of reflected light but 'Hardness' is defined as a mineral's resistance to scratching. The mineral class that accounts for more than 90% of the Earth's crust are the silicates.
It is true that a rock can be composed of almost entirely one mineral. However, it is not true that all minerals have cleavage and color is not a reliable technique for the identification of minerals.
Learn more about Chemistry here:https://brainly.com/question/36629312
#SPJ11
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
Everything in the world is made of matter except you??
TrueFalse
Answers
Answer:
False.
Explanation:
Humans are made of matter too :)
Refer to the following balanced equation in which ammonia reacts with nitrogen monoxide to produce nitrogen and water.
4NH3(g) + 6NO(g) -> 5N2(g) + 6H2O(l)
How many moles of NH3 are necessary to produce 0.824 mol N2?
___mol NH3
(Note the question must be answered with three significant figures)
Answers
Answer: 0.659 mol
Explanation:
From the equation, we can tell that for every 4 moles of ammonia consumed, 5 moles of nitrogen are produced.
This means the answer is 0.824(4/5)=0.659 mol
Part A
Write a hypothesis about what will happen to the air in the plastic bottle when its temperature is
decreased. What relationship do you expect to find between temperature and volume?
Answers
It is hypothesized that as the temperature of the air inside the plastic bottle decreases, the volume of the air will decrease as well, in accordance with Charles's Law and the contraction of the plastic bottle.
Hypothesis:
Based on the ideal gas law and the behavior of gases, it is hypothesized that when the temperature of the air inside a plastic bottle is decreased, the volume of the air will decrease as well. This hypothesis is rooted in the understanding that gases tend to contract and occupy less space when their temperature decreases.
According to Charles's Law, which states that the volume of a gas is directly proportional to its temperature when pressure is held constant, it is expected that as the temperature of the air in the plastic bottle decreases, the gas molecules will lose kinetic energy, resulting in a decrease in their average speed. This decrease in speed will lead to a decrease in collisions between the gas molecules and the walls of the container, causing the air to occupy less volume.
Furthermore, since the air is trapped inside a plastic bottle, the decrease in temperature is expected to cause the plastic to contract slightly, exerting additional external pressure on the gas molecules and further reducing the volume they occupy.
For such more questions on temperature
https://brainly.com/question/30668924
#SPJ8