what is a limitation of using a chemical formula, such as C6H12O6, to represent a compound?
A.The chemical formula does not show the types of elements that make up the compound.
B.The chemical formula does not show how the atoms are connected to one another.
C.The chemical formula does not show the number of atoms of each element in a molecule.
D.The chemical formula does not show the chemical symbols of the elements in the compound.
Answers
Answer:
B
The chemical formula does not show how the atoms are connected to one another
Answer:
b
Explanation:
edge 2020
Related Questions
What do the orbital shapeshave to do with the spacial arrangement of any covalently bonded atoms?
Answers
Orbital shape has everything to do with the spatial arrangement of covalently bonded atoms.
In chemistry, orbitals are the regions where electrons are found orbiting around the nucleus of an atom.
The shape of the orbital is determined by the Schrödinger equation, which is a fundamental equation in quantum mechanics.
The spatial arrangement of any covalently bonded atoms is dictated by the orbitals involved in the bond.
The hybridization of orbitals occurs in the bonding process.
The orbitals combine to form new hybrid orbitals with different shapes, which determine the spatial arrangement of atoms.
These hybrid orbitals include sp, sp2, and sp3 orbitals, which correspond to different bond angles and geometries.
In conclusion, the shape of the orbitals affects the spatial arrangement of covalently bonded atoms.
Hybrid orbitals are formed when the orbitals combine, and these hybrid orbitals determine the spatial arrangement of the atoms.
To know more about spatial arrangement visit;
https://brainly.com/question/28321158
#SPJ11
The atomic number of calcium is 20. This number means that calcium has 20 protons. The atomic mass of calcium is 40. How many neutrons does calcium have? (Remember: protons + neutrons = atomic mass.)
ASAP NOW PLS
Answers
Answer:
Otherwise, positive charge means that the element lost an electron and negative charge means it gained an electron. (3) The atomic mass is equal to the sum of the number of protons and number of neutrons. An atom of the calcium-40 isotope has 20 neutrons in its nucleus.
How to write ionic compund formulas
ex Na+ & F-
Answers
You with writing ionic compound formulas. An ionic compound consists of a positive ion (cation) and a negative ion (anion) bonded together through electrostatic forces. To write the formula of an ionic compound, you need to balance the charges of the cation and anion to ensure the compound is neutral.
In your example, you have a sodium ion (Na+) and a fluoride ion (F-). The sodium ion has a positive charge of +1, while the fluoride ion has a negative charge of -1. To write the formula for the ionic compound formed by these two ions, you simply combine them in a way that balances their charges. Since the charges are already equal and opposite, you just need to put them together:
Na+ & F- → NaF
The resulting ionic compound is sodium fluoride (NaF). To write formulas for other ionic compounds, follow the same process:
1. Identify the cation and anion involved.
2. Determine the charges of each ion.
3. Balance the charges by adjusting the number of ions as needed.
4. Write the formula, placing the cation first followed by the anion.
Remember to always ensure that the charges are balanced, and the resulting compound is neutral. This method will allow you to write ionic compound formulas effectively.
Learn more about ionic compound here
https://brainly.com/question/28759239
#SPJ11
What is one element that is found in the human body, air, or the universe ?
Answers
Answer:
Hydrogen
Explanation:
It is pretty much present everywhere :)
How many moles are in 1. 82 x 10^20 atoms of silver?
Answers
1.82x10^20 atom Ag x mole
———————————————- =
1 6.023x10^23 atom
= 0.000302 moles
how many moles of nickel(ii) iodide, nii2, are present in a sample that contains 7.18 moles of iodide ions?
Answers
The number of moles of nickel(II) iodide (NiI2) present in a sample containing 7.18 moles of iodide ions is 3.59 moles.
To calculate the moles of nickel(II) iodide, we need to consider the stoichiometry of the compound. The formula of nickel(II) iodide is NiI2, indicating a 1:2 ratio of nickel to iodide ions. Therefore, for every 2 moles of iodide ions, we have 1 mole of nickel(II) iodide.
Given that the sample contains 7.18 moles of iodide ions, we can calculate the moles of nickel(II) iodide using the stoichiometric ratio. Moles of NiI2 = Moles of I- / 2. Plugging in the values, we find that Moles of NiI2 = 7.18 moles / 2 = 3.59 moles. Hence, there are 3.59 moles of nickel(II) iodide in the sample.
To know more about, stoichiometry, click here https://brainly.com/question/28780091
#SPJ11
What is the ratio of aluminum atoms to oxygen atoms in aluminum oxide?
Answers
Answer:
Al2 O3 or 2:3
Explanation:
The aluminum oxide consists of two elements, which are oxygen atoms and aluminum atoms, and there are 2 aluminum atoms and 3 oxygen atoms in aluminum oxide. The ratio from aluminum atoms to oxygen atoms is 2:3.
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, the ratio of aluminum atoms to oxygen atoms in aluminum oxide is 2:3.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond. The ratio of aluminum atoms to oxygen atoms in aluminum oxide is 2:3. Aluminium oxide is an ionic compound.
Therefore, the ratio of aluminum atoms to oxygen atoms in aluminum oxide is 2:3.
To learn more about chemical compound, here:
brainly.com/question/26487468
#SPJ6
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this
information, what can Derek infer about horses and donkeys?
Horses and donkeys cannot survive in the same
environment.
Horses and donkeys produce fertile offspring.
Horses and donkeys are members of the same
population.
Horses and donkeys are members of different
populations.
Answers
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this information, Derek can infer that horses and donkeys are members of different populations.
Since horses and donkeys are different species, they belong to different populations. A population refers to a group of individuals of the same species that live in the same area and can interbreed. While horses and donkeys can mate, their offspring, known as mules, are usually infertile.
This means that mules cannot produce offspring of their own, which indicates that horses and donkeys are not members of the same population. In contrast, if they were members of the same population, they would be able to produce fertile offspring. Therefore, Derek can infer that horses and donkeys are members of different populations.
Learn more about mules here:
https://brainly.com/question/15852289
#SPJ11
Consider the following molecules and ions to answer the following questions: i). N2 ii). CN iii). O2 iv). F2 a. Which one has a bond order of 3?. b. Rank them in order of increasing stability. c. Which one is polar?
Answers
CN and N2 have a bond order of 1.5, while O2 has a bond order of 1 and F2 has a bond order of -1. The order of increasing stability is F2, O2, N2, and CN. CN is the only polar molecule among the four.
The bond order of a molecule can be determined by the difference between the number of bonding and anti-bonding electrons divided by 2. Using this formula, we can calculate the bond orders of the given molecules and ions:
i) N2: The Lewis structure of N2 shows a triple bond between the two nitrogen atoms. There are 5 bonding electrons and 2 anti-bonding electrons, giving a bond order of (5-2)/2 = 1.5.
ii) CN: The Lewis structure of CN shows a triple bond between the carbon and nitrogen atoms. There are 6 bonding electrons and 3 anti-bonding electrons, giving a bond order of (6-3)/2 = 1.5.
iii) O2: The Lewis structure of O2 shows a double bond between the two oxygen atoms. There are 6 bonding electrons and 4 anti-bonding electrons, giving a bond order of (6-4)/2 = 1.
iv) F2: The Lewis structure of F2 shows a single bond between the two fluorine atoms. There are 3 bonding electrons and 5 anti-bonding electrons, giving a bond order of (3-5)/2 = -1.
In terms of stability, a higher bond order generally indicates greater stability because there are more bonds holding the atoms together. Therefore, the order of increasing stability would be F2, O2, N2 and CN.
To determine polarity, we need to look at the electronegativity difference between the atoms in the molecule. A polar molecule has a positive and negative end due to an unequal sharing of electrons.
In N2 and F2, the two atoms are the same and therefore have no electronegativity difference, making them nonpolar. In O2, the electronegativity difference between the two oxygen atoms is small and the molecule is also nonpolar. However, in CN, the electronegativity difference between carbon and nitrogen is significant enough to make the molecule polar.
In summary, CN and N2 have a bond order of 1.5, while O2 has a bond order of 1 and F2 has a bond order of -1. The order of increasing stability is F2, O2, N2, and CN. CN is the only polar molecule among the four.
for more such question on bond order
https://brainly.com/question/30461012
#SPJ11
What is the formula for ammonium
Answers
Answer:
The formula for ammonium is NH₄⁺
Balance the folowing equation.
S + 02
ws
SO:
Answers
Answer:
s+o2=so2
Explanation:
i hope it will help u
25. Which is an irreversible process?
1) mixing of two gases by diffusion
2) Evaporation of water at 373k and 2atm
pressure
3) Dissolution of Nacl in water
4) All are correct
(S
Answers
The answer is 1) mixing of two gases by diffusion.
Once 2 gases are mixed by diffusion, they cannot be obtained from the mixture of gases. Hence, it is considered to be an irreversible process.
20g of H2O of dissolve 7.6g of salt at 25°C. What is the solubility of the salt in g\100g of water at that temperature.
Answers
The solubility of the salt in grams per 100 grams of water at 25°C is 38 g/100g. This means that at the given temperature, 38 grams of the salt can dissolve in 100 grams of water.
To determine the solubility of the salt in grams per 100 grams (g/100g) of water, we need to calculate the mass of the salt dissolved in 100 grams of water at 25°C. Given:
Mass of water (H2O) = 20g
Mass of salt dissolved = 7.6g
To find the solubility, we divide the mass of the dissolved salt by the mass of water and multiply by 100:
Solubility = (Mass of salt dissolved / Mass of water) * 100
Plugging in the values:
Solubility = (7.6g / 20g) * 100
Solubility = 38 g/100g
For more question on solubility
https://brainly.com/question/23946616
#SPJ8
What is always true according to the Law of Conservation of Matter?
Answers
Answer:
same amount of matter exists before and after the change—none is created or destroyed
Explanation:
example
water will have the same mass when it freezes into ice
sciencingcom
Who developed the orbital model of the atom?
O J.J. Thomson
O Dmitri Mendeleev
O Ernest Rutherford
ONiels Bohr
Answers
Answer:
Ernest Rutherford
Explanation:
that's I think
how is this equation read?
4Na (1) +Mn(SO 4 ) 2 (aq) Mn (3) +2Na 2 SO

Answers
Question 5 of 25
Which two phrases best describe vascular tissue in plants?
A. Contain root hair cells and guard cells
B. Cover the outside of plants
c. Contain cells that connect to form pipes
D. Transport water and food
SUBMIT
Answers
Limiting line of balmer series result when electron jumps from orbitImmersive Reader
A) 3 to 2
B) ∞ to 2
C) 4 to 5
D) ∞ to 1
Answers
Answer:
A) 3 to 2
Explanation:
The Balmer series refers to all transition series of spectral emission lines of the hydrogen atom in which an electron moves from any higher energy level within the atom down to the energy level with principal quantum number n=2.
The Balmer series was originally calculated using the Balmer formula, this is an empirical equation first deduced by Johann Balmer in 1885.
Hence, a transition from n=3 level to n=2 level is in the Balmer series.
How many atoms are in 75.4 moles of Copper?
Answers
Answer:
No = Avagardro's number = 6.02 x 10^23
No.of atoms = no.of moles x No x atomicity
= 75.4 x 6.02 10^23 x 1
= 453.908 x 10^23 atoms .
I onlyI and II onlyII onlyII and III onlyI and III onlyIII only

Answers
The species that act as acid are the ones that donates H⁺ in the reaction.
Since we are working with an equilibrium, we have to consider both directions.
From left to right, we have H₂O turning into OH⁻ because it lost one H⁺, so H₂O is acting as an acid in the forward reaction.
From right to left, we have CH₃NH₃⁺ tunrning into CH₃NH₂ because it lost one H⁺, so CH₃NH₃⁺ is acting as an acid in the backwards reaction.
So, the species that act as acids are H₂O and CH₃NH₃⁺, I and II only.
describe how the following factors can influence the change in the volume of water on either side of a selectively permeable membrane:
A) The chemical nature of the solute and the solvent
B) Differences in solute or solvent molecule size
C) Size (diameter) of the dialysis tubing pore
D) Changes in osmolarity
how might the difference in osmolarity and tonicity inside and outside of dialysis tubing influence the overall rate of diffusion of different molecules?
Answers
The difference in osmolarity and tonicity inside and outside of dialysis tubing can influence the overall rate of diffusion of different molecules.
The chemical nature of the solute and solvent can influence the change in the volume of water on either side of a selectively permeable membrane because different solutes and solvents have different affinities for water. If the solute has a higher affinity for water than the solvent, water will move towards the side with the solute in order to create an equilibrium.
Differences in solute or solvent molecule size can also influence the change in the volume of water. Larger molecules may not be able to pass through the membrane as easily as smaller molecules, which can result in a buildup of solute or solvent on one side of the membrane and a change in the volume of water. The size (diameter) of the dialysis tubing pore can also influence the change in the volume of water. If the pores are too small, water may not be able to pass through as easily, leading to a buildup of water on one side of the membrane.
Changes in osmolarity can also influence the change in the volume of water. Osmolarity is a measure of the concentration of solute in a solution. If there is a difference in osmolarity between the two sides of the membrane, water will move towards the side with the higher osmolarity in order to create an equilibrium.
If there is a higher osmolarity or tonicity on one side of the membrane, water will move towards that side in order to create an equilibrium. This can result in a faster rate of diffusion for certain molecules, as they will be able to move more easily through the membrane.
To learn more about Osmolarity :
https://brainly.com/question/14814464
#SPJ11
In Niels Bohr’s model of the atom, how are electrons configured?
Answers
In Niels Bohr’s model of the atom, electrons are configured in a series of concentric shells around the nucleus. The shells are numbered, with the shell closest to the nucleus being numbered one, and each succeeding shell numbered two, three, and so on.
The electrons in the innermost shell have the lowest energy, while those in the outermost shell have the highest energy. Each shell can hold a certain number of electrons. The first shell can hold up to two electrons, the second shell up to eight electrons, and the third shell up to 18 electrons. Electrons fill the shells in a specific order, following the Aufbau principle. The principle states that electrons will occupy the lowest available energy level before filling higher levels. Electrons in the same shell have the same energy. Electrons in different shells have different amounts of energy, which corresponds to the distance of the shell from the nucleus. When an electron absorbs energy, it can move to a higher energy level. When an electron loses energy, it can move to a lower energy level. Electrons can also move between atoms, which is the basis of chemical reactions.For such more question on concentric shells
https://brainly.com/question/13569827
#SPJ8
How much water would need to be added to 750 ml of a 2.8 m hcl solution to make a 1.0 m solution?
Answers
1.35 Litres of water need to be added to 750 ml of a 2.8 m hcl solution to make a 1.0 m solution.
Use the following relation:
M1V1=M2V2
Where M is molarity, V is volume and 1 is initial and 2 is the final conditions. Solving for V(2)
M1=2.8 M,V1=750 mL;M2=1.0 M
(2.8 M)×(750 mL)=(1.0 M)×V2
V2=(2.8 M)×(750 mL)(1.0 M) = 2100 mL = 2.1 L
Therefore, Volume of water to be added =2.1 L−0.75 L=1.35 L
Learn more about the Molarity with the help of the given link:
https://brainly.com/question/19517011
#SPJ4
The process in which solvent molecules surround solute molecules is called...
Select one:
a. concentration.
b. distillation.
c. filtration.
d. solvation.
Answers
Answer: Its D. I got it correct
Explanation:
The general word is solvation.
Answer:
D.Solvation
Explanation:
Solvation is the process in which solute particles are surrounded by solvent molecules.
hope this time I am correct
What is the ph of a buffer system prepared by dissolving 10. 70 grams of nh4cl and 35. 00 ml of 12 mol l-1 nh3 in enough water to make 1. 000 l of solution? kb = 1. 80 × 10-5 for nh3.
Answers
9.577 is the ph of a buffer system prepared by dissolving 10. 70 grams of nh4cl and 35. 00 ml of 12 mol l-1 nh3 in enough water to make 1. 000 l of solution.
Mass ,NH4Cl = 10.7 g
Volume ,NH3 = 35 mL = 0.035 L
Molarity NH3 = 12 M
no. moles of NH4Cl = mass/molar mass
Molar mass, NH3 = 53.49 g/mol
no. moles NH4Cl = 10.7/53.49
no. moles NH4Cl; n_acid = 0.2 mol
no. moles NH3 = Volume × molarity
no. moles of NH3 = 0.035 × 12
no. moles NH3; base = 0.42 mol
kb = 1.8 × 10^(-5)
Pkb = -log kb
Pkb = -log(1.8 × 10^(-5))
Pkb = 4.745
Pka = 14 - Pkb
Pka = 14 - 4.745
Pka = 9.255
PH = Pka + log(n_base/n_acid)
PH = 9.255 + log(0.42/0.2)
PH = 9.577
To know more about ph visit : https://brainly.com/question/13043236
#SPJ4
which subatomic particles are located in the nucleus of a ca-40 atom?
1) electrons and neutrons
2) electrons and protons
3) neutrons and protons
4) neutrons, protons, and electrons
Answers
Answer:
3) neutrons and protons
Explanation:
I just completed Grade 11 Chemistry with a 97% and I'm still under the impression that all atoms have only neutrons and protons in their nuclui.
I apologize if this is a question above my current education.
if 0.00849 moles of bai₂ makes 0.00283 moles of precipitate and 0.00630 moles of na₃po₄ makes 0.00315 moles of precipitate, which reactant is limiting?
Answers
In the given statement, the reactant that is limiting is BaI₂.
What is BaI₂?
BaI₂ is a chemical compound composed of barium and iodine. It is an ionic compound, meaning that the barium and iodine atoms are held together by electrostatic forces. BaI₂ is a yellow-brown colored solid at room temperature. It is used in the production of certain semiconductor materials and in medicine as a contrast agent for imaging studies.
The reactant is limiting because the amount of BaI₂ that is needed to produce the same amount of precipitate (0.00315 moles) is double the amount of Na₃PO₄ that is needed to produce the same amount of precipitate (0.00283 moles). This means that for the reaction to proceed, there must be twice as much BaI₂ as Na₃PO₄, and since there is only 0.00849 moles of BaI₂, the reaction will be limited by the amount of BaI₂ available.
What is Precipitation reaction?
A precipitation reaction is a type of chemical reaction in which two solutions are mixed together to form an insoluble salt, which is referred to as the precipitate. This type of reaction is often used to identify the presence of certain ions in solution, as the formation of a precipitate indicates that the ions in question are present.
To know more about precipitation reaction,
https://brainly.com/question/28903618
#SPJ4
9) what is the empirical formula of a compound containing 47.37 g carbon, 10.59 g hydrogen, and 42.04 g oxygen?
Answers
The empirical formula of the compound containing 47.37 g carbon, 10.59 g hydrogen, and 42.04 g oxygen is CH₂O.
To determine the empirical formula, we need to find the simplest whole-number ratio of the elements present in the compound. The given masses of carbon, hydrogen, and oxygen can be converted into moles by dividing them by their respective atomic masses. The molar ratios of the elements can then be determined by dividing the number of moles of each element by the smallest number of moles calculated.
In this case, the molar ratios are approximately 1:2:1, indicating that the compound contains one carbon atom, two hydrogen atoms, and one oxygen atom. Therefore, the empirical formula of the compound is CH₂O, representing the simplest ratio of elements present in the compound.
To know more about CH20 click here:
brainly.com/question/29797602
#SPJ11
13. What would be the boiling point in degrees celcius of the solution in number 12?
(Kb of water is 0-.512 °C/m)
Answers
The boiling point of the solution in number 12 would be 100.624 °C.
How do we determine the value?The boiling point of a solution depends on many factors, including the concentration of the solute and the nature of the solvent. The value you provided (Kb of water = 0.512 °C/m) is the molal boiling point elevation constant, which is used to calculate the change in boiling point of a solvent when a solute is added. Using the molar boiling point to multiply the number ascribed to the degree Celsius of the solution which is 12 plus the boiling point of water. which is 100..
Therefore, the correct answer is as given above
learn more about boiling point: https://brainly.com/question/40140
#SPJ1
Please help me. No links or false answers!! Correct the following expressions into the correct scientific notation.
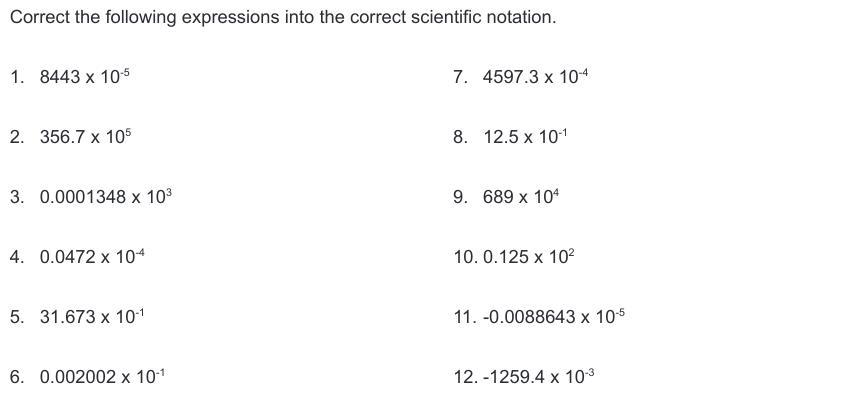
Answers
The aim of the scientific notation is to be able to reduce the bulk of the figures that we have to deal with.
What is the scientific notation?The aim of the scientific notation is to be able to reduce the bulk of the figures that we have to deal with. Thus, when we apply the scientific notation, we reduce the digits that we are dealing with to manageable number and this makes it easy for us to convey scientific information.
Let us now write the correct expression of the given numbers in scientific notation:
1) 8.443 * 10^-1
2) 3.567 * 10^8
3) 1.348 * 10^-1
4) 4.72 * 10^-6
5) 3.1673 * 10^0
6) 2.002 * 10^-4
7) 4.5973 * 10^-1
8) 1.25 * 10^0
9) 6.89 * 10^6
10) 1.25 * 10^1
11) -8.8643 * 10^-8
12) - 1.2594 * 10^0
Learn more scientific notation:https://brainly.com/question/18073768
#SPJ1