Vinegar (acetic acid) and baking soda (sodium bicarbonate) react in an endothermic reaction to produce sodium acetate, carbon dioxide, and water. Which statement justifies classifying the reaction as endothermic?
A. The reaction produces water.
B. The bonds in both the reactants and products are covalent bonds.
C. The reaction needs a catalyst.
D. The total potential energy of the products is less than the total potential energy of the reactants.
Answers
Answer: The total potential energy of the products is less than the total potential energy of the reactants
Explanation:
A chemical reaction in which the heat of the system gets increased as it absorbs the heat from the surroundings and transfers it to the system is called an endothermic reaction.
The potential energy of the reactants is more than the products.
What happens in endothermic reactions?Vinegar and baking soda react by absorbing the heat from the system and reacting to produce carbon dioxide, sodium acetate and water.
In this type of reaction, the reactants absorb heat resulting in increased potential energy and is high compared to that of the products produced.
Therefore, option D is correct.
Learn more about endothermic reactions here:
https://brainly.com/question/25923072
Related Questions
A. frequency C. wavelength G What do we call the height of a wave? B. amplitude D. speed
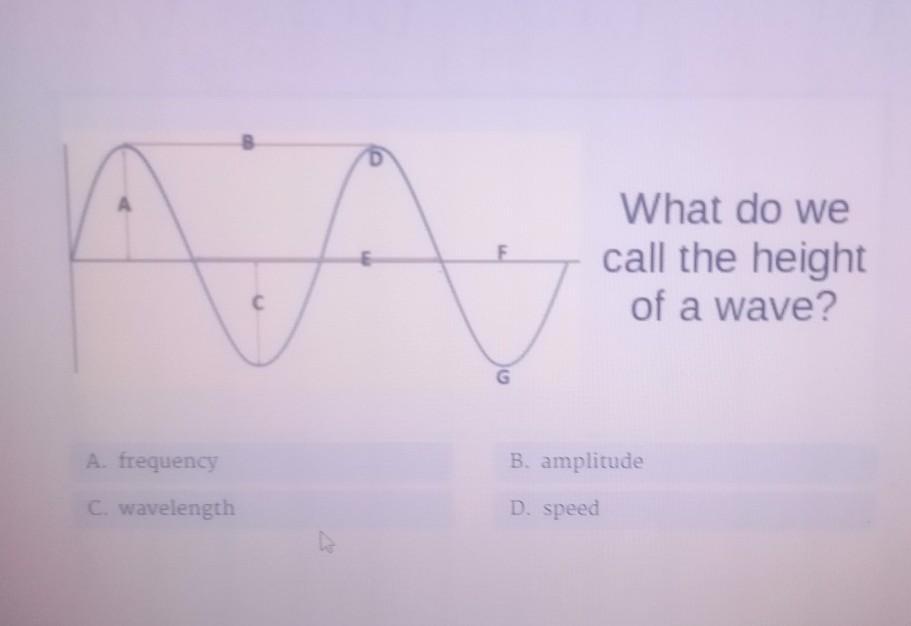
Answers
Answer:
Explanation:
The height of the wave is called the amplitude.
so the correct answer is B.
the specific heat capacity of glass is 0.20 cal/(g°C). If 30 cal of heat is added to an unknown mass of glass, the temperature rises by 150°C. what is the mass of the glass?
Answers
The mass of the glass is 1 gram.
What is specific heat capacity?
This refers to the amount of heat in joules (J) absorbed per unit mass (kg) of the material when its temperature rises 1 K (or 1 °C), and its units are J/(kg K) or J/(kg °C).
From the question:
cp= 0.20 cal/(g°C)
T = 150°C
heat added = 30 cal
Solution:To find the mass of the glass, you can use the formula:
mass = heat added / (specific heat capacity x temperature change)
Substituting the given values:
mass = 30 cal
(0.20 cal/(g°C) x 150°C)
mass = 30
(0.20 x 150)
mass = 30
30
mass = 1 g
Hence, the mass of the glass is 1 gram.
Learn more about specific heat capacity on
https://brainly.com/question/1747943
#SPJ1
gravity and magnetism are examples of what?
Answers
Answer:
hyposthesis
Explanation:
true or false: only mutations that alter amino acid residues in the active sites of enzymes affect the function of the enzyme.
Answers
only mutations that alter amino acid residues in the active sites of enzymes affect the function of the enzyme. False.
Mutations that alter amino acid residues in the active sites of enzymes can certainly affect the function of the enzyme, but mutations that occur in other regions of the enzyme can also have an impact on its function. Enzymes are large, complex molecules with a specific three-dimensional structure that is critical to their function. Changes in the amino acid sequence of an enzyme can affect its structure and, in turn, its function. Mutations in regions of the enzyme that are not part of the active site can affect the stability or conformation of the enzyme, which can impact its ability to bind substrates, catalyze reactions, or undergo allosteric regulation.
Additionally, mutations that alter amino acid residues in domains or regions of the enzyme that are involved in interactions with other proteins or cofactors can also affect the function of the enzyme. For example, mutations in regulatory domains of an enzyme can affect its ability to be activated or inhibited by other proteins or small molecules.
Therefore, while mutations in the active sites of enzymes can certainly affect their function, mutations in other regions of the enzyme can also have significant effects on their activity, specificity, and regulation.
To know more about enzyme
brainly.com/question/31385011
#SPJ11
Which formula contains 2 non metals

Answers
Answer:
The answer is SiO2
Explanation:
Because nonmetals are those who gain electrons and form ve ions
causes of majimaji rebellion results
Answers
Answer:
The war was triggered by a German policy designed to force the indigenous population to grow cotton for export and lasted from 1905 to 1907, during which 250,000–300,000 died. After the scramble for Africa among the major European powers in the 1880s, Germany reinforced its hold on several formal African colonies.
Explanation:
please give me brainlist and follow
Electrochemistry-Related Question:
The answer is "A"
but I don't understand this question, I need explanation

Answers
The only incorrect statement in the diagram is (d) Cr202-7 can be used in aqueous H2SO4.
A detailed explanation of the Standard Electrode PotentialOption (d) is incorrect because the half-cell reaction involving Cr2O7^2- and H+ (aq) produces H2CrO4, which can decompose in acidic solutions, leading to inaccurate results. Therefore, Cr2O7^2- should not be used in aqueous H2SO4 for the quantitative estimation of Fe(NO3)2-.
Learn more about Electrode Potential here:
https://brainly.com/question/17362810
#SPJ1
The Only incorrect statement is option C
What is electrochemistry?Electrochemistry is a branch of chemistry that deals with the study of the relationship between electrical energy and chemical reactions.
It involves the study of the behavior of electrons and ions in chemical reactions that occur in a solution or at the interface between two different phases, such as a solid electrode and a liquid electrolyte.
We can see that it is better to use HCl instead of the use of the H2SO4 acid as we have in the options.
Learn more about electrochemistry:https://brainly.com/question/16912983
#SPJ1
The sturdy wall outside of a skyscraper could be most easily compared to
Question 14 options:
A cell membrane
B mitochondrion
C chloroplast
D cell wall
Answers
GIVING BRAINIEST FIVE STARS AND HEART!
Please define the following term in your own words!
law of conservation of matter
Answers
Answer:
Matter cannot be created destroyed
Explanation
Matter can change form through physical and chemical changes, but through any of these changes, matter is conserved. The same amount of matter exists before and after the change—none is created or destroyed. This concept is called the Law of Conservation of Mass.
Draw the major organic product of the following friedel–crafts alkylation. (an excess of benzene is present. ) do not draw any aluminum byproducts
Answers
The Friedel–crafts alkylation provide a nice way of attaching an alkyl group to an aromatic compound.
What is Friedel–crafts alkylation?The Friedel–crafts alkylation occurs when an alkyl group is added to an aromatic compound.
The reaction mechanism commences with the attack of an alkyl halide on an aromatic substrate using AlCl3 as a catalyst. This question is incomplete henece we can not draw the structure of the product. The alkyl portion of the alkyl halide is now attached to the aromatic compound at te end of the reaction.
Learn more about Friedel–crafts alkylation: https://brainly.com/question/26874245
7. If two elements are in the same column, why does the one that is higher have a stronger
coulombic attraction?
Answers
The element that is in a higher column will have a stronger coulombic attraction because this property is dependent on the size of the charge.
What is an Element?This is known as any substance which can't be broken down into smaller parts through a chemical means and examples include helium, magnesium etc.
On the periodic table, we have two broad divisions in which the rows are called period while the column is referred to as group. As we move up the column, the coulombic attraction increases.
This is so because a high number of protons will lead to a corresponding increase in the positive charge. This then improves the strength of the nucleus and it is able to pull the electrons further away from it thereby making it the most appropriate reason.
Read more about Coulombic attraction here https://brainly.com/question/12246130
#SPJ1
A student adds 3.00 g of dry ice (solid CO2) to an empty balloon. What will be the volume of the balloon at STP after all the dry ice sublimes (converts to gaseous CO2)?
Answers
3 grams will produce approximately 1.53 liters of gaseous phase of CO2
Which percentage is in the appropriate range for a snack to be considered a “good source” of nutrients?
15 percent
35 percent
40 percent
50 percent
Answers
Answer:
15
Explanation:
Which of the following is an alkaline earth metal. Hh
Answers
Answer:
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium, magnesium, calcium, strontium, barium, and radium.
Hope this helped!
What volume (mL) of 7.48x10 -2 M phosphoric acid can be completely reacted with 115 mL of .244 M sodium
hydroxide? Hint: balance equation
Answers
Answer:125ml - volume of phosphoic acid
Explanation:
The equation of the reaction is
H3PO4 + 3 NaOH ---> Na3PO4 + 3H2O
Such that I mole of H3PO4 reacts with 3 moles of NaOH to produce the products above.
Given that
M1= Molarity of phosphoric acid = 7.48x10^-2 M
n1= number of moles of phosphoric acid = 1
V1=? ml
M2= Molarity of Sodium hydroxide =0.244 M
V2= volume of sodium hydroxide= 115ml
n2=number of moles of sodium hydroxide = 3
Using the Dilution equation formulae,
M1V1/n1 = M2V2/n2
7.48X10-2 x V1 /1= 0.244M x 115ml/3
V1=0.244Mx115mlx1/0.0748M X 3
V1= 28.06/0.2244= 125.04ml
V1=125.04ml rounded up to 125ml
Now that you know a little more about chemical reactions, think about the Rumpelstiltskin story. In the fairy tale, one substance was involved in a process—spinning on the wheel—and then a different substance was made. Straw turned into gold. If the fairy tale were real, then that change would be called a chemical reaction. The substance that Rumpelstiltskin started with (straw) was different from the substance he ended with (gold). In real life, substances can change into other substances, but only in a fairy tale could straw turn into gold. Why? (Hint: Think about what gold is made of.) What is the scientific reason that straw cannot be turned into gold?
Answers
Gold is a chemical element which is produced by the collision of supernovas and stellar remnants.Processes like atomic fusion or fission only can produce gold.
What are nuclear reactions?It is a process by which two nuclei or sub -atomic particles collide to produce one or more nuclides. For reactions to be called nuclear it is necessary for it to transform at least one nuclide .
In nuclear reaction, total energy is conserved.The energy is released in in forms of kinetic energy, emission of high energy photons and some products which are meta-stable.
There are 4 types of nuclear reactions :
1) fission
2)fusion
3) nuclear decay
4) transmutation
Generally, in nuclear reactions,there is a heavy nucleus and light bombarding particle which produces 2 new particles heavier nucleus and lighter ejected particle.
Learn more about nuclear reactions ,here:
https://brainly.com/question/12649087
#SPJ2
sodium chloride is a buffer creating an injectable solution that is isotonic. group of answer choices true
Answers
Yes, the statement is True i.e. Sodium chloride is a buffer creating an injectable solution that is isotonic.
A buffer solution, also referred to as a pH buffer or hydrogen ion buffer, is an aqueous mixture of a weak acid and its conjugate base, or vice versa. The pH scarcely changes at all when a small amount of a strong acid or basic is added to it. Buffer solutions are used in a wide range of chemical processes to keep pH values almost constant. Buffering is used by many living systems to regulate pH in the natural world. For instance, the bicarbonate buffering system regulates the pH of blood, and bicarbonate also acts as a buffer in the ocean. Because of a chemical equilibrium between the weak acid HA and its conjugate base A, buffer solutions are resistant to pH change: HA ⇌ H+ + A− According to Le Chatelier's principle, an equilibrium between a weak acid and its conjugate base shifts to the left when hydrogen ions (H+) are supplied. Because of this, despite the supply of strong acid, the concentration of hydrogen ions increases less than anticipated.
To know more about buffer please refer: https://brainly.com/question/22821585
#SPJ4
By adding electrons to an uncharged object, the object *
becomes negatively charged
becomes positively charged
becomes energized
changes color
Answers
Answer:
becomes negatively charged
Explanation:
this is because when an object receives electrons it becomes negatively charged and the object that loses electrons becomes positively charged.
Answer:
First one because by adding more electrons it makes something more negative. So it becomes negatively charged
which molecules of the following gases will have the greatest average kinetic energy? 1. h2 at 0.5 atm and 298 k 2. co2 at 1 atm and 298 k 3. n2 at 1 atm and 298 k 4. all of the molecules have the same average kinetic energy. 5. he at 0.1 atm and 298 k
Answers
The kinetic energy of molecules depends on absolute temperature. So here since all the temperature are equal, all the molecules will have same average kinetic energy. So option 4 is right.
Kinetic energy of a molecule of a gas depends on the movement of the molecule. It is governed by the kinetic gas equation. The kinetic energy and temperature is related by the equation
KE = \(\frac{3}{2}\) nRT
n is the number of moles
R is universal gas constant
T is the absolute temperature
KE is directly proportional to the temperature and increases and decreases with it. Here all gases exists at the same temperature. Pressure does not have any effect on the kinetic energy of gases.
So option 4 will be the correct answer.
For more information regarding Kinetic energy of gases, kindly refer
https://brainly.com/question/2696774
#SPJ4
Balance the equation Mg+CO2 - - - - - -) MgO+c
Answers
Answer:
2Mg (s) + CO₂ (g) → 2MgO (s) + C (s)
General Formulas and Concepts:
Chemistry - Reactions
Reaction PredictionBalancing RxN'sExplanation:
Step 1: Define RxN
Mg (s) + CO₂ (g) → MgO (s) + C (s)
Step 2: Balance RxN
2Mg (s) + CO₂ (g) → 2MgO (s) + C (s)
We need to balance the number of O's on both sidesIf we balance the O's, we will also need to balance the Mg'sBased on the electron configuration of the two atoms, predict the ratio of metal cationic (+) atom to nonmetal anionic (-) atom in the compound. 1522s22p63523p64sl Potassium 1$22s22p63s23p5 Chlorine
Answers
The metal cationic (+) atom to nonmetal anionic (-) atom ratio in the compound formed between Potassium and Chlorine is 1:1.
What is a compound?A compound is made up of two or more atoms that are chemically combined together. In this case, we have the atoms; Potassium and Chlorine.
The electronic configuration of the atoms is not shown here but the metal cationic (+) atom to nonmetal anionic (-) atom ratio in the compound formed between Potassium and Chlorine is 1:1.
Learn more about chemical compounds:
brainly.com/question/12166462
#SPJ1
3) How many milliliters of 0.210 M NaC2H3O2 are needed to supply 12.2 g NaC2H3O2?
Answers
For this question we will be using two main formulas:
1. Finding the number of moles of the compound, since we have mass and the molar mass of NaC2H3O2 is 82.03g/mol
Molar mass = mass/number of moles
We already have molar mass and mass
82.03 = 12.2g/number of moles
n = 12.2/82.03
n = 0.15 moles of NaC2H3O2
2. Now we can find the volume in mL, by using the Molarity formula, which is:
Molarity (or concentration) = number of moles/volume in Liters
We already have the molarity and number of moles
0.210 M = 0.15 moles/V in L
V = 0.15/0.210
V = 0.714 L or 714 mL
Which 5-carbon intermediate of the citric acid cycle is converted to a 4-carbon molecule with the release of carbon dioxide?
Answers
The alpha- ketoglutarate is the 5-carbon intermediate of the citric acid cycle which converted to a 4-carbon molecule with the release of carbon dioxide
The first decarboxylation reaction in Krebs cycle ( known as citric acid cycle ) is catalyzed by isocitrate dehydrogenase in which one six carbon atoms compound isocitrate is decarboxylated to yield five carbon atom containing alpha ketoglutaric acid.
The alpha- ketoglutarate is the 5-carbon intermediate of the citric acid cycle which is salt of alpha ketoglutaric acid. The last reaction in the citric acid cycle produces a product that is a substrate for the first reaction of the citric acid cycle.
To learn more about citric acid cycle
https://brainly.com/question/11459709
#SPJ4
Which of the following best illustrates a natural process acting as a constructive force
Answers
Answer:
Wind depositing sand to build up sand dunes.
Explanation:
Calculate the molarity of a solution that has 2.3m of NaOH dissolved in 1.3L
Answers
The molarity of the NaOH solution is 1.77 M.
Molarity is defined as the number of moles of solute dissolved in one liter of solution. To calculate the molarity of the NaOH solution, we need to first determine the number of moles of NaOH present in the solution.
The formula to calculate the number of moles is:
moles = mass ÷ molar mass
where:
mass is the mass of the solute (in grams)
molar mass is the molar mass of the solute (in g/mol)
In this case, we are given the molarity and volume of the solution, but we need to first find the number of moles of NaOH.
To do this, we can rearrange the formula for molarity:
Molarity = moles ÷ volume
Rearranging the formula to solve for moles gives us:
moles = Molarity x volume
Substituting the given values gives us:
moles = 2.3 mol/L x 1.3 L
moles = 2.99 mol
Therefore, the solution contains 2.99 moles of NaOH.
Finally, we can calculate the molarity of the solution by dividing the number of moles by the volume of the solution in liters:
Molarity = moles ÷ volume
Molarity = 2.99 mol ÷ 1.3 L
Molarity = 1.77 M
The molarity of the NaOH solution is 1.77 M.
To know more about molarity, visit;
https://brainly.com/question/17138838
#SPJ11
BRAINLIEST! PLEASE HELP! To plate gold onto a base metal, the overall reaction is Au+3(aq) + 3e− → Au(s). How many seconds would it take at a current of 3.5 amps to plate out 2.50 grams of gold?
a. 349 s
b. 1049 s
c. 9.5 x 10^-4 s
d. 17.4 s
Answers
The required time it would take at a current of 3.5 amps to plate out 2.50 grams of gold is 1,049 seconds.
How do we calculate the required time?According to the faraday's second law, equation to calculate the time will be given below:
W = ZIt, where
W = deposit mass = 2.50 grams
Z = equivalent mass / 96500 = 65.6 / 96500 = 0.000679
I = current = 3.5 amp
t = required time = ?
On putting values in above equation, we get
t = (2.50) / (0.000679)(3.5)
t = 1,054 sec = 1,049 s (approx)
Hence required seconds to plate out 2.50 g of gold is 1,049 sec.
To know more about faradays law, visit the below link:
https://brainly.in/question/1850079
#SPJ1
Based on your understanding of how bond types influence a material’s properties, identify each of the following compounds as being made of ionic, covalent, or metallic bonds.
Steel:
Propane:
Calcium chloride:
Water:
A 2-column table with 3 rows. Column 1 is labeled Substance with entries Steel, Propane, Calcium Chloride, and Water. Column 2 is labeled properties with entries Conducts electricity as solid and liquid; low melting point; conducts electricity when dissolved in water, and solid form is brittle, melts at 0 degrees Celsius.
Answers
Atoms with vastly different electronegativity levels can form ionic bonds. Atoms with minor variances in electronegativity form covalent connections with one another.
How may the type of atom-atom bonding affect a material's properties?Crystal cleavage and symmetry are two further features influenced by chemical bonding. In general, stronger chemical bonds lead to greater hardness, higher melting and boiling temperatures, and smaller coefficients of expansion. Stronger chemical bonds between atoms make them more difficult to separate.
What are the four main kinds of bonds and how do they come into being?The valence and bonding preferences of an atom's constituents can typically be used to predict a solid's qualities. Ionic, covalent, metallic, and molecular bonds are the four basic types of bonds covered here. ice and other solids with hydrogen bonds.
To know more about ionic, covalent, or metallic bonds visit :-
https://brainly.com/question/3162660
#SPJ1
I'm very confused please help

Answers
Answer:
the food chain you mean
Explanation:
pollution than mosquitoes then alligators
If there are 6,500 tcf of reserves of natural gas in the world and we are consuming 123 tcf per year, how long until we run out?.
Answers
According to the given statement 52.8 years long until we run out.
The correct option is A.
What do you mean by a natural gas?A blend of hydrocarbon-rich gases makes up natural gas. Methane, nitrogen, carbon dioxide, and other gases are all naturally occurring in the environment. Deep inside the earth, amid other beds of solid and liquid hydrocarbons like coal and crude oil, lie natural gas reserves.
What is the source of natural gas?Renewable natural gas, which is created from decomposing organic materials, must also be compressed or liquefied in order to be used as a transportation fuel. Methane makes up the majority of the odorless, gaseous combination of hydrocarbons known as natural gas (CH4).
To know more about Natural gas visit:
https://brainly.com/question/14870839
#SPJ4
The complete question is -
If there are 6,500 tcf of reserves of natural gas in the world and we are consuming 123 tcf per year, how long until we run out?.
A-52.8 years
B-77 years
C-90 years
D-105 years
Match each part of the atom with its identity from the list below.

Answers
Answer:
Nucleus: Choice C
Electron: Choice E
Proton: Choice A
Neutron: Choice B
Energy Level: Choice D
Explanation:
1. Nucleus contains the protons and neutrons.
2. Electrons surround the nucleus and have a negative charge.
3. Protons are positively charged and found in the nucleus.
4. Neutrons have a neutral charge and are found in the nucleus.
5. The energy level refers to the electron orbital.