Answers
The answer is true. The yeast engages in aerobic respiration in the presence of oxygen, converting carbohydrates (a source of sugar) into carbon dioxide and water.
The yeasts do fermentation in the absence of oxygen, turning carbohydrates into alcohol and carbon dioxide. Aerobic growth is substantially better for yeast cells. They cannot execute oxidative phosphorylation in anaerobic conditions, therefore they must manufacture all of their ATP through less effective glycolysis. When there is water, sugar, and no oxygen present, yeasts perform fermentation. The yeasts engage in anaerobic respiration, which implies their metabolic processes do not include the consumption of oxygen.
To learn more about oxygen, click here.
https://brainly.com/question/13370320
#SPJ4
Related Questions
Solids and liquids have higher densities than gases.
Answers
Answer:
yes
Explanation:
a tire will burst if the air inside it reaches a pressure greater than 1.4 x 10^3 kpa. at what temperature will the tire burst if it has a volume of 30L and contains 2.5 mol of air? assume that the air behaves as an ideal gas. assuming that these values are representative, do you need to worry about your car tire bursting from overheating of they are in good condition?
Answers
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
To determine the temperature at which the tire will burst, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for temperature, we have:
T = PV / (nR)
Given that the pressure threshold for bursting is 1.4 x 10^3 kPa, the volume is 30 L, and the number of moles of air is 2.5 mol, we can substitute these values along with the ideal gas constant R = 8.314 J/(mol K) into the equation.
T = (1.4 x 10^3 kPa) * (30 L) / (2.5 mol * 8.314 J/(mol K))
Converting kPa to Pa and L to m^3, and simplifying the equation, we find:
T ≈ 20,993 K
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
For more question on temperatures
https://brainly.com/question/4735135
#SPJ8
What type of molecule is acetylacetone?

Answers
Answer:
here is the answer
Explanation:
ketone is the answer
A chemistry student weighs out 0.0838 g of phosphoric acid (H₂PO4), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled
water. He plans to titrate the acid with 0.2000M NaOH solution.
Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.
Answers
The volume of NaOH the student will need to reach the final equivalence point = 12.8ml
The balanced equation shows the reaction between phosphoric acid and sodium hydroxide.
3NaOH + H3PO4 ====> Na3PO4 + + 3H2O
This equation implies that for 1 mole (or 1n) of phosphoric acid, 3 moles of NaOH will be required.
nNaOH = 3 x nH3PO4
MV (NaOH) = 3 x mass of H3PO4
molar mass
Now let's do the calculations to find the volume of NaOH to find the equivalence point. As we know the molar mass of phosphoric acid is 97.994 g/mol.
V (NaOH) = 3 x mass of H3PO4
molar mass x M (NaOH)
= 3 x 0.0838
97.994 x 0.2000
V (NaOH) = 12.8x 10-³L x 1000ml/ 1L
The volume of NaOH = 12.8ml
To see more numerical on phosphoric acid, click on:
https://brainly.com/question/14812997
#SPJ9
Oxygen is represented by a red sphere. Nitrogen is represented by a blue sphere. Hydrogen is represented by a white sphere. In the reactants there are five molecules that contain two white spheres and four molecules that contains one red and one blue sphere. In the products there are two molecules that contain two blue spheres, four molecules that contain one red and two white spheres, and one molecule that contains two white spheres.
What is the chemical formula for the limiting reactant in the reaction shown?
Answers
Answer:
5H2 + 4NO = 2N2 +4H2O + H2
Explanation:
Reactants:
Five molecules that contains two hydrogen = 5H2
Four molecules that contains one nitrogen and one oxygen = 4NO
Products:
Two molecules that contains two nitrogen = 2N2
Four molecules that contains one oxygen and two hydrogen = H2O
One molecules that contains two hydrogen = 1H2 or H2
The balanced chemical equation for the reaction will be: 5 H₂ + 4 NO → 2 N₂ + 4 H₂O + H₂, and there is no limiting reactant in this reaction.
To write the balanced chemical equation for the reaction, let's first identify the reactants and products based on the information provided:
Reactants:
5 molecules contain 2 white spheres (Hydrogen): 5 H₂
4 molecules contain 1 red (Oxygen) and 1 blue (Nitrogen) sphere: 4 NO
Products:
2 molecules contain 2 blue spheres (Nitrogen): 2 N₂
4 molecules contain 1 red (Oxygen) and 2 white (Hydrogen) spheres: 4 H₂O
1 molecule contains 2 white spheres (Hydrogen): 1 H₂
Now, let's write the balanced chemical equation by ensuring that the number of atoms of each element is the same on both sides of the equation:
Reactants: 5 H₂ + 4 NO
Products: 2 N₂ + 4 H₂O + 1 H₂
The balanced chemical equation for the reaction is:
5 H₂ + 4 NO → 2 N₂ + 4 H₂O + H₂
Now, let's determine the limiting reactant:
From the balanced equation, we can see that the stoichiometric ratio between H₂ and NO is 5:4. This means that for every 5 molecules of H₂, we need 4 molecules of NO for the reaction to proceed completely.
Since we have 5 molecules of H₂ and 4 molecules of NO, both reactants are present in the exact stoichiometric ratio required for the reaction. Therefore, neither H₂ nor NO is in excess, and both will be fully consumed during the reaction. As a result, there is no limiting reactant in this reaction.
To know more about limiting reactant here
https://brainly.com/question/33417913
#SPJ2
--The given question is incomplete, the complete question is
"Oxygen is represented by a red sphere. Nitrogen is represented by a blue sphere. Hydrogen is represented by a white sphere. In the reactants there are five molecules that contain two white spheres and four molecules that contains one red and one blue sphere. In the products there are two molecules that contain two blue spheres, four molecules that contain one red and two white spheres, and one molecule that contains two white spheres. What is the chemical formula for the limiting reactant in the reaction shown? chemical formula: Write the balanced chemical equation for the reaction, using lowest whole-number coefficients."--
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
If you burn yourself in lab you should?
A. See the nurse after class
B. Tell the instructor
C. See a doctor after school
D. Apply first aid yourself
Answers
Answer:
B. Tell the instructor
Explanation:
Always to the instructor about any accidents happens in a lab.
Answer:
B
Explanation:
Just helping out:)
What problems did Haber face? Think about equilibrium!
In your
explanation include the words nitrogen, hydrogen, ammonia, equilibrium, and
pressure.
Answers
how old is Billie Eilish
Answers
Answer:
21
Explanation:
........................
Answer: 21
Explanation:
Balance the chemical equation below using the smallest possible whole number stoicbiometric coefficients
CH3 (CH2)4 CH,(g) +02(g) → CO2(g) + H2O(g)
Answers
The balanced chemical equation is the representation of the elements with the same proportion of elements on both sides. The balanced equation is, 2CH₃(CH₂)₄CH₃ + 19O₂ → 12CO₂ + 14H₂O.
What is a balanced equation?An equation is said to be balanced if the number of atoms of the element on the right side is the same as that of the left side of the reaction.
The unbalanced reaction is:CH₃(CH₂)₄ CH₃(g) + O₂(g) → CO₂(g) + H₂O(g)
Here the number of carbon, hydrogen, and oxygen are unbalanced.
Coefficients are added before carbon, oxygen, and a hydrogen atom. First, 2 is added before the carbon on the reactant side and 12 at the carbon of the product side.
After this, the number of hydrogen is balanced by adding 14 on the product side followed by adding 19 on the oxygen of the reactant side.
The balanced equation: 2CH₃(CH₂)₄CH₃ + 19O₂ → 12CO₂ + 14H₂O
Therefore, 2CH₃(CH₂)₄CH₃ + 19O₂ → 12CO₂ + 14H₂O is the balanced equation.
Learn more about balanced equations here:
https://brainly.com/question/2396833
#SPJ1
A container holds one mole of a gas if the amount of gas is tripled how many molecules of gas will be in the container?
Answers
Answer:At the molecular level, the pressure of a gas depends on the number of collisions its molecules have with the walls of the container. If the pressure on the piston is doubled, the volume of the gas decreases by one-half. The gas molecules, now confined in a smaller volume, collide with the walls of the container twice as often and their pressure once again equals that of the piston.
Explanation:
Answer:
thanks
Explanation:
17.1 grams of magnesium metal burns in sulphur dioxide to form magnesium oxide and sulphur write a balanced equation for the reaction and calculate the mass of magnesium oxide and the mass of sulphur that forms.
Answers
Mass of MgO = 28.35grams
Mass of Sulphur = 11.29 grams
Explanations:The balanced chemical equation between magnesium metal and sulphur dioxide is given as:
\(2Mg+SO_2\rightarrow2MgO+S\)Determine the moles of magnesium
Mole = mass/molar mass
Mole of Mg = 17.1/24.305
mole of Mg = 0.704moles
According to stoichiometry, 2 moles of Mg produces 2 moles of MgO, hence the required mass of MgO will be:
\(\begin{gathered} Mass\text{ of MgO}=0.704\times40.3 \\ Mass\text{ of MgO}=28.35grams \end{gathered}\)Similarly, 2moles of Mg produces 1 mole of sulphur, hence the mass of sulphur produced is;
\(\begin{gathered} Mass\text{ of S}=\frac{1}{2}\times0.704\times32.065 \\ Mass\text{ of S}=11.29grams \end{gathered}\)Hence the mass of magnesium oxide and the mass of sulphur that forms is 28.35grams and 11.29grams
3. Tom wants to make some copper oxide. Sally suggests he could make it by burning copper metal in air.
a) Why is Sally wrong?
Answers
Answer:
Because burning copper in air won't oxidize it you need to leave it in contact with air only
Explanation:
atoms and ions are held together by..
A.) nuclear bonds
B.) Stick bonds
C.) physical bonds
D.) Chemical bonds
Answers
Answer:
chemical bonds
Explanation:
The atoms in chemical compounds are held together by attractive electrostatic interactions known as chemical bonds. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The ions are held together in a regular spatial arrangement by electrostatic forces.
I need help please:
Zootopia:
This movies theme is on stereotypes within culture and the influence police have on the Publics perception. Explain how this can happen and it’s impact on civil and criminal justice.
Answers
Answer:
down below
Explanation:
The police's influence can happen based on a response to a problem. An example is when the police are called because of a thief child. If the police handles the situation correctly-using reasonable force, if necessary, and reading them their rights- the public will perceive the police in a good light or way. If the police use gross misconduct and do not go by the book, then they will be perceived as an enemy or in a bad light or way.
How many moles of MgS are in 100.g MgS?
Answers
The number or amount of moles of magnesium sulfide in 100g of MgS is 1.774 moles.
How to calculate number of moles?The number of moles in a substance can be calculated by dividing the mass of the substance by its molar mass as follows:
Mole is the base unit of amount of substance i.e. the amount of substance of a system which contains exactly 6.02214076 × 10²³ elementary entities.
no of moles = mass ÷ molar mass
According to this question, 100g of magnesium sulfide is given. The molar mass of magnesium sulfide is 56.38 g/mol.
moles = 100g ÷ 56.38g/mol
moles = 1.774 moles
Learn more about moles at: https://brainly.com/question/26416088
#SPJ1
Which element best conducts electricity?
argon
copper
nitrogen
oxygen
Answers
Answer:
I think it is copper
Explanation:
.........................
Answer:
copper
Explanation:
QUICK I WILL CHOOSE BRAILEST
What is convection?
1. Transfer of heat through objects touching source
2. Transfer of heat through direct physical contact
3. Transfer of heat through electromagnetic waves
4. Transfer of heat through the movement of particles
Answers
Answer:
C
Explanation:
Answer:
4. Transfer of heat through the movement of particles
Explanation:
because it goes in a circular motion
11. The major constituents of the Earth's current atmosphere are
a. moslty oxygen with some CO2
b. 78% oxygen, 21% nitrogen
C.78% nitrogen, 21% oxygen
12. The Earth's primitive atmosphere
a. was primarily hydrogen and helium, like the giant planets'
b. escaped due to the Earth's rapid loss of heat from its interior
C. was mostly carbon dioxide, since plants hadn't yet formed to add oxygen
13. The Earth's magnetic field stretches out in an area called the
A.Magnetosphere
b. Troposphere
C. Atmosphere
Answers
Answer:
C, C, A
Explanation:
11. C
12. C
13. A
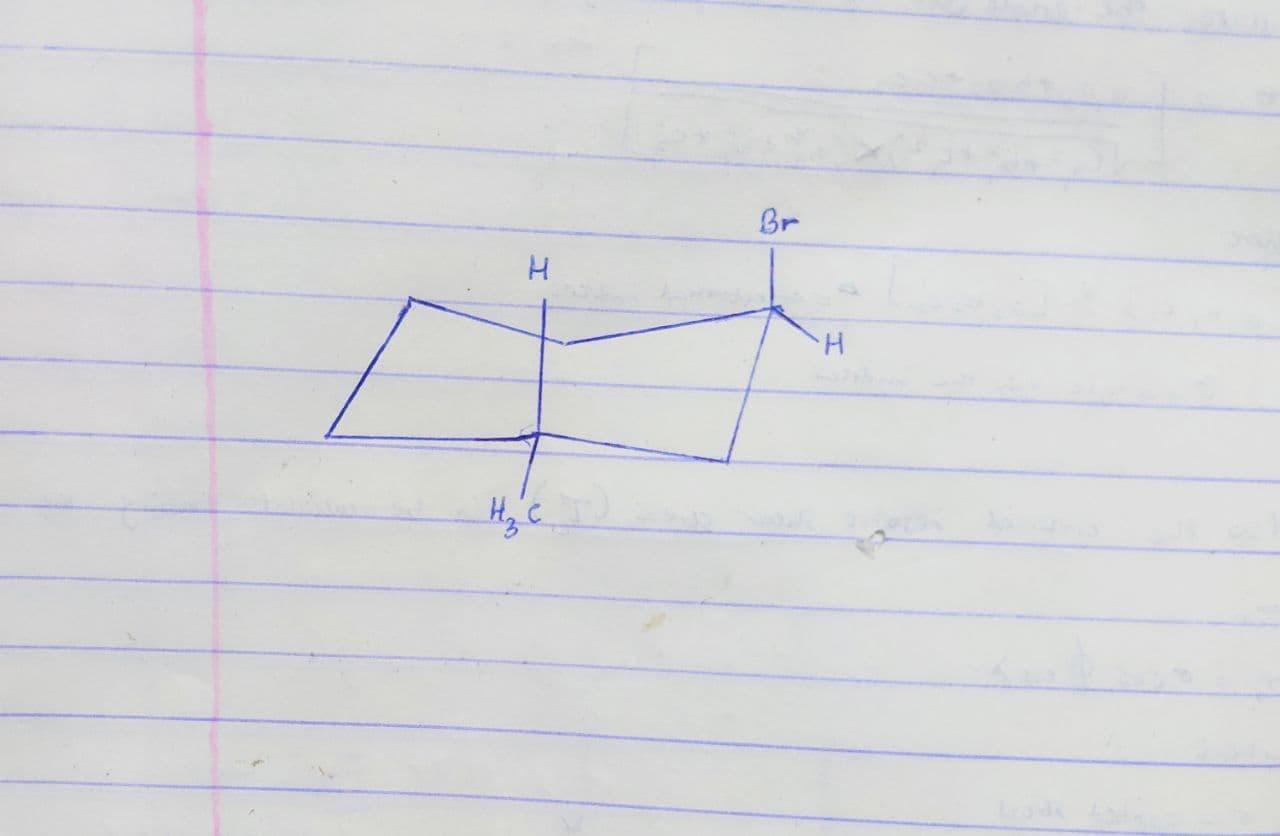
Complete a chair conformation of trans-1-bromo-3-methylcyclohexane by placing the hydrogen, bromine, and methyl groups in the appropriate positions.
Place the bromine on the carbon that is more to the right.
Answers
Answer:
Explanation:
The most highly stable cycloalkane is cyclohexane. It does not suffer from an angle or torsional strain, and it has the appropriate stability as chain alkanes. Because of the peculiar conformation it takes, this stability leads to the cyclohexane conformation popularly known as the "chair" conformation.
However, from the information given;
The chair conformation structure of trans-1-bromo-3-methylcyclohexane is carefully drawn and the substituents are appropriately attached in the image below.
A gas expands and does PV work on its surroundings equal to 322 J. At the same time, it absorbs 132 J of heat from the surroundings. Calculate the change in energy of the gas. Note: PV work means work done by a changing volume against constant pressure. Enter your answer in scientific notation.
Answers
From the calculations, the change in energy is - 190 J.
What is the first law of thermodynamics?From the first law of thermodynamics, the energy is neither created nor destroyed but is transformed from one form to another.
From the law;
U = q + w
U = internal energy
q = heat
w = work
Since work is done on the surroundings and the gas absorbs heat then;
U = 132 J - 322 J
U = - 190 J
Learn more about thermodynamics:https://brainly.com/question/1368306
#SPJ1
The q,ß-unsaturated ketone A exchanges hydrogen atoms with deuterium at the two positions shown below to give product B. Provide a detailed, stepwise mechanism for the following transformation using proton transfer reactions.
Use curved arrows to show the movement of electrons.
O+D --------> D20 (solvent) D D
Answers
Complete Question
The complete question is shown on the first uploaded image
Answer:
The step wise mechanism is shown on the second uploaded image
Explanation:


Look at the attachment below.

Answers
Sally is wrong because copper is less electropositive than hydrogen, thus, can not displace hydrogen from dilute acids.
The reactions to prepare copper (ii) chloride are:
the chlorination of copper sulfide at a high temperature
reaction of copper (ii) oxide with dilute hydrochloric acid
The equations of the given reactions are as follows:CuS + Cl₂ ---> CuCl₂ + SCuO + 2HCl ----> CuCl₂ + H₂O
What are reactive metals?Reactive metals are metals that readily give up their electrons to form positive ions.
Reactive metals displace hydrogen from dilute acids. They are found in group 1A and 2A of the periodic table. Copper is not a reactive metal and will not displace hydrogen from acids.
Learn more about reactive metals at: https://brainly.com/question/20273277
#SPJ1
Sally is wrong because copper chloride is not made from the reaction of copper and dilute hydrochloric acid.
2. Copper (ii) chloride can be prepared as follows:
reacting copper (ii) oxide with dilute hydrochloric acidsingle replacement reaction of copper sulfide and chlorine gas at a high temperature3. the equations of the reaction are:
CuO + 2HCl ----> CuCl₂ + H₂OCuS + Cl₂ ---> CuCl₂ + SWhat are single replacement reactions?Single replacement reactions are reactions in which a more reactive atom replaces another atom in a compound.
An example of a single replacement reaction is the reaction of chlorine gas with copper sulfide at high temperatures to form copper chloride.
Learn more about single replacement reaction at: https://brainly.com/question/20216315
#SPJ1
Which of these statements best explains why chemistry is reliable?
Answers
Answer:
It gives the same result when an experiment is repeated.
Explanation:
Below are the possible answers to the question:
It is biased.
It cannot be verified.
It cannot add new evidence to existing evidence.
It gives the same result when an experiment is repeated.
The correct answer would be that it gives the same result when an experiment is repeated.
If a reaction is conducted in chemistry and certain results are obtained, once a detailed procedure of the experiment is known along with all the chemicals involved, such reaction/experiment can be repeated anywhere in the world and the same result would be obtained.
The repeatability of experiments always makes the experiments to be reliable. Hence, chemistry is reliable because it gives the same result without any variation when experiments are repeated under similar conditions.
Suppose you pull off the broken tungsten filament in a burned-out incandescent
light bulb. You take its mass and find it to be 0.100 grams. How many atoms of
tungsten are in this filament?
5.44 x 10-
1.84 x 1025
3.28 x 1020
6.022 x 1023
Answers
Molar mass of tungsten =183u
No of moles
0.1/1830.0055molNo of atoms
Moles×Avagadro no0.0055(6.022)10²³0.0033×10²³3.3×10²⁰Option C
Consider the following reaction:Mg + Br2 → MgBr2Which of the following statements is true?Group of answer choicesThe bromine atom is losing electrons; therefore, it is reduced.The bromine atom is gaining electrons; therefore, it is oxidized.The magnesium atom is gaining electrons; therefore, it is oxidized.The magnesium atom is losing electrons; therefore, it is oxidized.none of these
Answers
The First step is to write and balance our reaction:
Mg + Br2 → MgBr2
As we can see Mg and Br2 have a charge equal to zero. Therefore, on the right side, we must have a zero electrical charge too.
--------------
The second step is to analyze what happens to each of them when the reaction occurs.
Mg) On the left, its oxidation state is 0, then in MgBr2, Mg has a +2 charge. So, we can say that if the atom goes from 0 to +2, it loses electrons (loses negative charges or gain positive charge). We can also say that Mg oxidized.
Until here, the atom that loses electrons gets oxidized.
Br)On the left, Br starts with 0, then in MgBr2, it has -1. So we can say that Br gained electrons (gain negative charge). We can also say that Br is reduced.
-------------
The 3rd step is to find the right statement.
Answer: The magnesium atom is losing electrons; therefore, it is oxidized.
Describe echolocation. Give an example of an animal that uses echolocation.
Answers
dolphin and whales use echolocation
Use the dropdown menus to complete the following statements explaining the concept of freezing point depression.
Hint: It may be helpful to review the introduction section of FPD in your lab manual.
In a pure solvent ____
The presence of solute ___
The solvent's freezing point ___
Answers
In a pure solvent, the freezing point is a constant. The presence of solute causes the freezing point of the solvent to decrease. The solvent's freezing point is depressed.
A solution's freezing point is lower than that of the pure solvent's. This indicates that freezing can only take place when a solution is lowered to a lower temperature than the pure solvent. Road salting in water is a relatively prevalent example of this occurrence in daily life. At 0°C, pure water freezes. In a pure solvent, the freezing point is the temperature at which the solvent will form a solid. The presence of solute particles interferes with the ability of the solvent molecules to form a solid, causing the freezing point to decrease. The solvent's freezing point is thus lowered or "depressed" by the addition of solute particles.
To learn more about solvent click here https://brainly.com/question/30452436
#SPJ4
solution solution solution

Answers
Answer:
Oxygen present in food items makes then rancid due to the presence of oils and fats. If the food is flushed with nitrogen, it prevents it from being oxidised (the nitrogen acts as an antioxidant).
Hope it helps ! :)
help me in my hw,wt is physical change and chemical change Answer it asap plz don't spam
Answers
Answer:
Sorry but i don't undertsnad the question.
Explanation:
Answer:
A physical change is a change to the physical—as opposed to chemical—properties of a substance. They are usually reversible. The physical properties of a substance include such characteristics as shape, color, texture, flexibility, density, and mass.
A chemical change happens when one chemical substance is transformed into one or more different substances, such as when iron becomes rust.
Do u want examples ?