True or False: The isotope with the greatest
abundance contributes to the atomic mass
reported on the periodic table more than
the isotope of lower abundance.
Answers
Answer:
True
Explanation:
Greater abundance means there is more of those isotopes, so they contribute to the average number more. The average number is the atomic mass.
Related Questions
What is the frequency of gravity
Answers
In general, gravitational wave frequencies are much lower than those of the electromagnetic spectrum (a few thousand hertz at most, compared to some 1016 to 1019 Hz for X-rays). Consequently, they have much larger wavelengths – ranging from hundreds of kilometres to potentially the span of the Universe.
can someone tell me what are advantages and disadvantages of factory farming
Answers
Answer:
It keeps prices down for consumers. ... It allows automation to help provide food resources. ... It improves production efficiencies. ... Factory farms make it possible for market variety in every season. ... A factory farm can be established almost anywhere. ... It can lengthen food availability
Explanation:
One of the main drawbacks to having a factory near a town is pollution. Factories generally produce a lot of waste, which could hurt people's health and the environment. This is especially true if the factory uses large amounts of chemicals. Toxic waste may end up polluting the air, water and soil.
Convert particles to moles: 8.3 x 10^20 atoms Cu
Answers
Answer:
\(\boxed {\boxed {\sf 0.0014 \ mol \ Cu}}\)
Explanation:
We are asked to convert particles to moles.
1. Avogadro's Number1 mole of any substance contains the same number of particles (atoms, molecules, formula units). This is Avogadro's Number or 6.022*10²³.
In this problem, the particles are atoms of copper. So, 1 mole of copper contains 6.022*10²³ atoms of copper
2. Convert Atoms to MolesUse Avogadro's Number to make a ratio.
\(\frac{ 1 \ mol \ Cu}{ 6.022*10^{23} \ atoms \ Cu}\)
We are trying to convert 8.3*10²⁰ atoms of copper to moles, so we multiply that value by the ratio.
\(8.3*10^{20} \ atoms \ Cu*\frac{ 1 \ mol \ Cu}{ 6.022*10^{23} \ atoms \ Cu}\)
The units of "atoms Cu" will cancel.
\(8.3*10^{20}\frac{ 1 \ mol \ Cu}{ 6.022*10^{23} }\)
Condense the expression into 1 fraction.
\(\frac{8.3 *10^{20}}{ 6.022*10^{23} } \ mol \ Cu\)
\(0.001378279641 \ mol \ Cu\)
3. RoundThe original measurement of atoms has 2 significant figures, so our answer must have the same. For the number we calculated that is the ten-thousandths place.
0.001378279641The 7 in the hundredth thousandth place tells us to round the 3 up to a 4.
\(0.0014 \ mol \ Cu\)
8.3*10²⁰ atoms of copper are equal to 0.0014 moles of copper.
Does fertilizer make a plant grow bigger?mention two variables. How change of one variable effects another one in investigation?
Trick question
Science
Answers
In scientific investigations, the effect of fertilizer on plant growth can be studied by examining various variables. Two key variables in this context are the presence or absence of fertilizer (independent variable) and the size or growth of the plant (dependent variable).
When investigating the effect of fertilizer on plant growth, the independent variable is the presence or absence of fertilizer. This variable is controlled by having two groups of plants: one group receiving fertilizer (experimental group) and another group without fertilizer (control group). By comparing the growth of these two groups, we can determine the impact of fertilizer on plant size.
The dependent variable, on the other hand, is the size or growth of the plant. This variable is measured or observed as the outcome of interest. In this case, it would be the height, weight, or overall size of the plants.
By systematically changing the independent variable (presence or absence of fertilizer), we can observe how it affects the dependent variable (plant growth). The experimental group receiving fertilizer is expected to show greater plant growth compared to the control group without fertilizer. This allows us to draw conclusions about the effect of fertilizer on plant growth.
However, it is important to note that the specific outcome may vary depending on other factors such as plant species, soil conditions, and environmental factors. Conducting a controlled experiment while considering these factors helps in obtaining more reliable results.
learn more about fertilizer here
https://brainly.com/question/14012927
#SPJ11
calculate mole fraction of benzene (70 g) having 30 gram of mass of carbon tetrahedral
Answers
Answer:
0.736
Explanation:
*I am not sure what you mean by "carbon tetrahedral". I'm assuming you are just referring to individual carbon.*
To find the mole percent, you need to (1) convert grams benzene and grams carbon to moles (via their molar masses) and then (2) calculate the mole fraction (via the mole fraction formula).
(Step 1)
Benzene = C₆H₆
Molar Mass (C₆H₆) = 6(12.01 g/mol) + 6(1.008 g/mol)
Molar Mass (C₆H₆) = 78.108 g/mol
70 g C₆H₆ 1 mole
--------------- x ------------------ = 0.896 mole C₆H₆
78.108 g
Molar Mass (C) = 12.01 g/mol
30 g C 1 mole
------------ x ----------------- = 2.50 mole C
12.01 g
(Step 2)
moles solute
Mole Fraction = ----------------------------------------------
moles solute + moles solvent
2.50 mole C
Mole Fraction = ------------------------------------------------- = 0.736
(0.896 mole C₆H₆ + 2.50 mole C)
Topic: Mass Balance. A company sells fishmeal to be used as a protein supplement in certain foods. The process consists of: a. Extraction of fish oil, stage in which a pasta is obtained that has 20% flour and 80% water. b. Drying of pasta in a rotary drum, which produces fishmeal with 40% humidity. How much pasta must be input to the process to produce 1000 kg ?
Answers
To produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta. To determine the amount of pasta required to produce 1000 kg of fishmeal, we need to consider the mass balance of the process. Let's break down the steps involved:
A. Extraction of fish oil:
The pasta obtained from the extraction stage contains 20% flour and 80% water. To calculate the amount of pasta, we need to determine the mass of flour and water in the pasta. Let's assume the total mass of the pasta is P kg.
Mass of flour = 20% of P = 0.2P kg
Mass of water = 80% of P = 0.8P kg
b. Drying of pasta:
During the drying stage, the pasta is dried in a rotary drum, resulting in fishmeal with 40% humidity. This means that the final fishmeal will contain 60% dry matter.
Let's assume the mass of the dried fishmeal is M kg.
Mass of dry matter = 60% of M = 0.6M kg
Since the dry matter in the fishmeal comes from the flour in the pasta, we can equate the mass of dry matter to the mass of flour:
0.6M kg = 0.2P kg
To produce 1000 kg of fishmeal, we want to find the corresponding value of P:
0.6M = 0.2P
P = (0.6M) / 0.2
P = 3M
Therefore, to produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta.
To know more about mass balance, click here, https://brainly.com/question/17014679
#SPJ11
Which element has the smallest atomio radius?
Answers
a 20.0 ml solution of naoh is neutralized with 32.5 ml of 0.200 m hbr. what is the concentration of the original naoh solution?
Answers
The concentration of the original NaOH solution is 0.325M. Molarity is sometimes referred to as substance, molarity, or concentration in terms of amount. It is a means to determine the concentration of a certain chemical species. In terms of quantity, it alludes to the material in a unit solution volume.
Balanced chemical equation:
\(1NaOH+1HBr\) ⇒ \(NaBr+H_{2} O\)
Given,
1). volume of HBr(V1) = 32.5ml
molarity of HBr(M1) = 0.200M
moles n1 =1
2). volume of NaOH(V2) = 20.0ml
molarity of NaOH(M2) =?
moles n2 = 1
Moles of NaOH = Moles of HBr
Molarity of HBr × Volume of HBr= Molarity of NaOH × Volume of NaOH
\(\frac{M1V1}{n1} = \frac{M2V2}{n2} \\\)
\(\frac{0.2*32.5}{1} = \frac{M2*20}{1}\)
M2 = 0.2*32.5/20
M2 = 6.5/20
M2 = 0.325M
Molarity of NaOH = 0.325M
the concentration of the original NaOH solution is 0.325M.
Learn more about solution here:
https://brainly.com/question/4448910
#SPJ4
Which of the following are not potential "chemical hazards"? antibiotic traces O pesticides animal feces oven cleaner
Answers
Answer:
antibiotic traces because they can always put more of something and less of something
Explanation:
Methods to prevent plastic pollution
Answers
exchange polyhene bag with jute bags as they are reuseable
recycle plactic things
Sounds with great amplitude are ____________
Answers
Answer: LOUDER
Explanation:
. A larger amplitude means a louder sound, and a smaller amplitude means a softer sound.
NEED HELP ASAP
Select all the correct answers.
Which of these are examples of mixing and separating?
removing seed casings from grains
a soda bottle bubbling when it is opened
a bright copper statue turning green from weathering
removing salt from seawater
water decomposing to oxygen and hydrogen
Answers
Answer:
the answer is
removing seed casings from grains
removing salt from seawater
Explanation:
This question is regarding mixing and separating such as a physical change.
a soda bottle bubbling is a chemical reaction
copper statue is a chemical reaction
water decomposing to oxygen and hydrogen is also a chemical reaction
this question is talking about mixing and seperating as a physical change
A carrot was cut into many pieces, and moved into a freezer what change is it
Answers
Answer:
Physical change
Explanation:
Cutting and freezing are both examples of physical changes that do not alter the chemical composition or properties of the object/substance.
Answer:
physical change
Explanation:
carrot lost its original shape
t a certain temperature, t k, kp for the reaction, h2(g) cl2(g) ⇌ 2 hcl(g) is 2.18 x 1042. calculate the value of δgo in kj for the reaction at 705 k.
Answers
The value of ΔG° in kJ for the reaction at 705 K is -1.60 x 10^6 kJ/mol.
To calculate the value of ΔG° in kJ for the reaction at 705 K, we need to use the following equation:
ΔG° = -RTln(Kp)
Where R is the gas constant (8.314 J/mol K), T is the temperature in Kelvin (705 K), and Kp is the equilibrium constant (2.18 x 10^42).
First, we need to convert the equilibrium constant from Kp to Kc, which can be done using the equation:
Kp = Kc(RT)^Δn
Where Δn is the difference in the number of moles of gas between the products and the reactants. In this case, Δn = 2 - 1 - 1 = 0, since there are 2 moles of gas on both sides of the equation.
Therefore, we can calculate Kc as:
Kc = Kp/(RT)^Δn
Kc = 2.18 x 10^42 / (8.314 J/mol K x 705 K)^0
Kc = 2.18 x 10^42
Now, we can plug this value into the equation for ΔG°:
ΔG° = -RTln(Kp)
ΔG° = -8.314 J/mol K x 705 K x ln(2.18 x 10^42)
ΔG° = -1.60 x 10^6 kJ/mol
Here you can learn more about ΔG°
https://brainly.com/question/13738716#
#SPJ11
1.Mitch weighs out 67 grams of potassium (K) to make a buffer. How many moles of potassium did Dr. Hellman weigh out?
2.Which statement is NOT true about a reaction rate?
Group of answer choices
Increases with increase in reactant concentration
Increases with increasing temperature
Is the speed at which product is formed
Is the rate at which reactant is used up
All of the answers are true
3.Which statement is NOT true about a catalyst?
Group of answer choices
Are not used up during a reaction
Increases the rate of the reaction
Lowers the energy of activation
Biological catalysts are called enzymes
Are used up during a reaction
Answers
Answer:
1. 1.72 moles of potassium.
2. All of the answers are true
3. Are used up during a reaction
Explanation:
Recall that the number of moles is obtained from;
Number of moles= Mass of potassium/ molar mass of potassium
Mass of potassium= 67 g
Molar mass of potassium= 39 gmol-1
Number of moles of K= 67 g/ 39 gmol-1
Number of moles = 1.72 moles of potassium.
2. When we look at all the options, we will realize that all the options are true. The rate of reaction doubles for each 10°C rise in temperature, increasing reactant concentration increases particle collision and ultimately increases the rate of reaction. Rate of reaction deals with rate of disappearance of reactants or rate of appearance of products.
3. Catalysts remain unchanged in a chemical reaction because they do not actually participate in the reaction. Hence they are not used up in any chemical reaction.
Silus wants to monitor the temperature of a reaction every 0.5 seconds for 30 minutes. He plans to generate a graph of the temperature values over time and insert the graph into a text document. Which pair of tools would be best for Silus to use?
Answers
Answer:
an electronic temperature probe and a computer
What happened to the Arctic Ocean during the past 40 years?(1 point)
Responses
It has gained more sea ice.
It has gained more sea ice.
It has grown smaller.
It has grown smaller.
It has become war
Answers
The molecular mass response of the seas to climate change has recently resulted in a decline inside the width, length, or volume of an Arctic sea ice.
Where on earth is ice growing more quickly?The Arctic often experiences end-of-summer minimum sea ice extents that are getting lower and less. The IPCC points to this shifting sea ice extent as evidence of a warming planet. However, Antarctica's extent of sea ice is expanding.
How has the planet become more compact?People may now quickly get information about events occurring in far locations because to the growth of the internet and the number of satellites. Consequently, the world has shrunk in size in modern times.
To know more about molecular mass visit:
https://brainly.com/question/18446366
#SPJ1
its not a question but i wanted to tell you guys that if you guyz like my answers then please don't forget to give like and follow at the same time. I am not forcing you to follow only if you guyz like my answer then like or follow. THANKS FOR READING..
Answers
Answer:
thank you for this oibnki
Answer:
Sure I'll go ahead and like your answers and follow you if that's what you wanted! :)
Explanation:
Fluorine is a non-metal. A fluorine atom has nine electrons. In terms of electrons. what happens when fluorine atoms react?
Answers
Fluorine will gain an electron to complete it's octet .
How?
Let's look at electronic configuration of Fluorine
Z=9Ec:-
\(\\ \sf\longmapsto 1s^22s^22p^5\)
So it needs 1 electron to complete octetidentify the acid associated with each conjugate base. nh3 choose... I⁻ ___
SO4²⁻ ___
Cl⁻ ___ OH⁻ ___
F⁻ ___
a. HF
b. Water
c. Sulfuric acid d. Hydronium ion e. HCI f. НІ g. Bisulfate ion
Answers
The acid associated with \(NH_3\) is \(NH_4^+\), with I- is HI, with \(SO_4^{2-}\) is \(HSO_4^-\), with Cl- is HCl, with OH- is \(H_2O\), and with F- is HF.
1. NH3: It is a base that accepts a hydrogen ion (H+) from an acid. \(NH_3 + H^+ --> NH_4^+\). The acid associated with \(NH_3\) is \(NH_4^+\).
2. I-: is a base that accepts a hydrogen ion (H+) from an acid. \(I^- + H^+ --> HI\) . The acid associated with I- is HI.
3. \(SO_4^{2-}\) : is a base that accepts a hydrogen ion (H+) from an acid. \(SO_4^{2-} + H^+ --> HSO_4^-\). The acid associated with \(SO_4^{2-}\) is \(HSO_4^-\).
4. Cl-: is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when Cl- accepts a hydrogen ion (H+). \(Cl^- + H^+ --> HCl\). The acid associated with Cl- is HCl.
5. OH-: It is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when OH- accepts a hydrogen ion (H+). \(OH^- + H^+ --> H_2O\). The acid associated with OH- is \(H_2O\).
6. F-: It is a base that accepts a hydrogen ion (H+) from an acid. \(F^- + H^+ --> HF\). The acid associated with F- is HF.
To learn more about acid click here https://brainly.com/question/29796621
#SPJ11
What does the number next to the element indicate?
Answers
Answer:
The atomic mass or atomic weight
Explanation:
what is the term called when it means to lacks properties of metal?
Answers
Answer:
The used term is nonmetal.
Explanation:
Nonmetals are elements that are not shiny or are not good conductors of electricity or heat.
The periodic table has approximately 23 nonmetals in it.
In the periodic table, we can find elements. These ones could be nonmetal and metals.
The elements that lack metallic properties are the nonmetals.
Metals are good conductors of heat and electricity, like Cu or Fe.
Although, nonmetals are not good conductors.
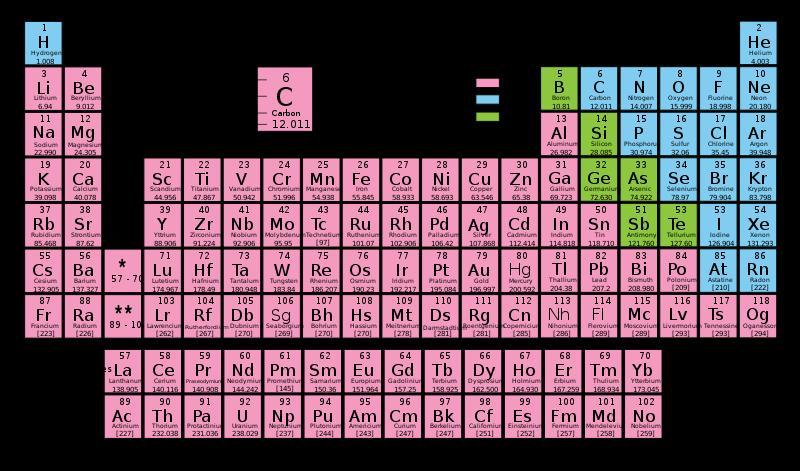
Hello I'm always here to help!!!
___________________________
A nonmetal is an element that lacks the majority of metal's characteristics. Nonmetals make up all of the elements in the shaded boxes. On Earth, many of the nonmetals are common elements. Nonmetals have physical traits that are diametrically opposed to metals. Solid nonmetals are dull, which means they aren't gleaming.
___________________________
Have a great day!!!!
what would you do if you left your working stock in room temperature
Answers
If I accidentally left my working stock in room temperature, there are a few steps that I would take in order to assess the damage and salvage the situation. First, I would check the expiration date of the stock to see if it was still viable before being exposed to room temperature. If it had expired, then there may not be much I could do to save it.
Assuming the stock was still viable, I would then inspect it for any signs of contamination or degradation.
If the stock had become contaminated or had started to degrade, then it would need to be discarded.
If the stock appeared to be still usable, I would then perform a series of tests to determine if it was still functional.
I would conduct a viability assay, such as a colony forming unit (CFU) assay or a growth curve analysis, to determine if the stock was still capable of growing and dividing.
If the stock was still functional, then I would use it for experiments as planned, but with the understanding that it may not perform as well as a freshly prepared batch.
To prevent this situation from happening in the future, I would take steps to label and store my stocks properly and to set reminders for myself to check on their status regularly.
It's always better to be safe than sorry when it comes to important reagents and stocks in the lab.
for more such question on temperature
https://brainly.com/question/1550666
#SPJ11
a flask contains 40 grams of solution that is 25% sugar by weight. how much sugar must be added to the flask so that the resulting solution is 40% sugar by weight?
Answers
To increase the resultant solution to 40% we need to add around 6 gram of sugar by weight.
To solve this problem, we need to find the weight of the sugar that must be added to the flask. Let's call this weight x.
We know that the total weight of the solution in the flask is 40 grams and that it is currently 25% sugar by weight. This means that there are 40 * 0.25 = 40*0.25=10 that is 10 grams of sugar in the flask.
We also know that the resulting solution should be 40% sugar by weight. This means that there should be 40 * 0.4 = 40*0.4=16 that is 16 grams of sugar in the solution.
Thus, we need to add 16 - 10 = 6 grams of sugar to the flask.
To know more about weight, click here,
brainly.com/question/2337612
#SPJ4
3.
Which of the following is not true for atoms? (4 points)
They combine to form elements and molecules.
They contain negatively charged electrons.
They are the building blocks of matter.
They are made up of elements.
Answers
Answer:
They are made up of elements.
Explanation:
An atom is the smallest particle of matter that still retains the property of the element.
Two or more atoms combine to form elements or compounds. Elements are formed by two or more similar atoms, while compounds are formed by two or more different elements.
Atoms are made up of subatomic particles, protons, electrons and neutrons. Electrons are negatively charged, protons are positively charged while neutrons have no charge.
I hope this help! :)
45 m/s = __________ km/hr
Answers
Answer:
162
Explanation:
(45/ 1000)*3600
=162
Which properties of metals and semi metals beat splits them away and explain the choice?
Answers
Metalloids, also known as semimetals, possess properties that lie between those of metals and nonmetals, differentiating them from both categories.
Metallic elements are typically good conductors of electricity and heat due to the presence of delocalized electrons in their outer energy levels. They also exhibit high luster and malleability, allowing them to be easily shaped into various forms. Conversely, semimetals demonstrate mixed properties, exhibiting some characteristics of metals and some of nonmetals. They possess moderate electrical conductivity, which can be enhanced or suppressed depending on external factors such as temperature or impurities. Semimetals also exhibit varying degrees of luster and may display brittle or semiplastic behavior.
The differentiation between metals and semimetals is primarily determined by their electronic structure and the position of their energy bands. Metals have a partially filled valence band and an empty conduction band, facilitating the movement of electrons, whereas semimetals have a partially filled valence band that overlaps with the conduction band, leading to their intermediate conductive properties.
In summary, the properties of metals and semimetals differ based on their electronic structure and physical characteristics. Metals possess high electrical and thermal conductivity, malleability, and luster, while semimetals exhibit intermediate conductivity and variable physical properties. The classification of elements as metals, semimetals, or nonmetals is based on their unique electronic band structure.
Learn more about semimetals from the given link: https://brainly.com/question/30161561
#SPJ11
If 2.22g of NaCl was recovered after the reaction of 0.050L of hydrochloric acid and 0.033L of sodium hydroxide. What was the molarity of the base used in this experiment?
Answers
The molarity of the base used in the experiment, which was determined based on the recovered NaCl and the volumes of hydrochloric acid and sodium hydroxide, was approximately 1.15 M.
To determine the molarity of the base used in the experiment, we need to use the stoichiometry of the balanced chemical equation and the given data.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl + NaOH → NaCl + H2O
First, we need to find the number of moles of NaCl produced. We can do this by using the given mass of NaCl (2.22 g) and its molar mass (58.44 g/mol):
moles of NaCl = mass of NaCl / molar mass of NaCl
moles of NaCl = 2.22 g / 58.44 g/mol
moles of NaCl = 0.038 moles
Next, we can use the stoichiometry of the balanced equation to determine the number of moles of NaOH that reacted. Since the mole ratio between NaCl and NaOH is 1:1, the number of moles of NaOH is also 0.038 moles.
Now, we can calculate the molarity of the base (sodium hydroxide) using the given volume of sodium hydroxide solution (0.033 L):
Molarity of NaOH = moles of NaOH / volume of NaOH solution
Molarity of NaOH = 0.038 moles / 0.033 L
Molarity of NaOH ≈ 1.15 M
Therefore, the molarity of the base used in the experiment is approximately 1.15 M.
For more such question on experiment. visit :
https://brainly.com/question/20639065
#SPJ8
_____ fatty acids consist entirely of carbon-carbon single bonds.
Answers
Saturated fatty acids
write an appropriate expression for and calculate a value of the entropy change ds associated with heating and expanding 3.00 moles of an ideal gas from a temperature of 298 k and a volume of 90.0 l to a temperature of 345 k and a volume of 120.0 l.
Answers
The appropriate expression for entropy change is ΔS = nR ln(V₂/V₁) + nCp ln(T₂/T₁).
The expression for calculating the entropy change (ΔS) of an ideal gas during a process involving temperature and volume changes is given by the equation above. Here, n represents the number of moles of the gas, R is the ideal gas constant, Cp is the molar heat capacity at constant pressure, V₁ and V₂ are the initial and final volumes respectively, and T₁ and T₂ are the initial and final temperatures respectively.
To calculate the value of ΔS, we substitute the given values into the equation:
ΔS = (3.00 mol)(8.314 J/(mol·K)) ln(120.0 L / 90.0 L) + (3.00 mol)(Cp) ln(345 K / 298 K)
The value of Cp depends on the specific gas involved, as it represents the molar heat capacity at constant pressure. The specific value of Cp would need to be provided to calculate the exact numerical value of ΔS.
By using the provided expression and plugging in the given values, along with the specific heat capacity value, you can calculate the entropy change associated with heating and expanding the ideal gas from the initial conditions to the final conditions.
To learn more about entropy change, here
https://brainly.com/question/28244712
#SPJ4