the water took longer to evaporate. what does this imply about the strength of the attraction of the molecules to each other?
Answers
The strength of attraction of the water molecules to each other is the hydrogen bonding.
The slowest evaporating liquid might be the water. Water 's hydrogen bonding, being the most powerful sort of intermolecular force, might be the toughest to triumph over to break out into the fueloline kingdom and could bring about the longest time. Hydrogen bonds do not form in all molecules because hydrogen can only form these bonds with highly electronegative atoms. It is a weak type of force that happens when a hydrogen atom is bonded to an atom which has a high electronegativity.
To learn more about hydrogen bonding check the link below:
https://brainly.com/question/1420470
#SPJ4
Related Questions
Biodiversity is important for the sustainability of ecosystems. However, many of the human actions that are aimed at growing communities are decreasing the biodiversity of these areas, leaving populations and ecosystems vulnerable. As human populations grow, the terrestrial and aquatic ecosystems they use may be transformed by the efforts of human beings and biodiversity losses typically accompany these processes. Which of these actions decrease biodiversity and threaten sustainability? Select ALL that apply.
A Invasive (non-native) species may outcompete native species for food and habitat. Invasive (non-native) species may outcompete native species for food and habitat.
B Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions. Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions.
C Pollution from building and commercialization can create health problems in exposed organisms. Pollution from building and commercialization can create health problems in exposed organisms.
D Decreased use of pesticides and fertilizers can allow for increased damage to plant life. Decreased use of pesticides and fertilizers can allow for increased damage to plant life.
E Habitat destruction reduces or eliminates the food resources and living space for most species. Habitat destruction reduces or eliminates the food resources and living space for most species.
Answers
The actions by humans that decrease biodiversity and threaten sustainability are;
Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions.Pollution from building and commercialization can create health problems in exposed organisms.Habitat destruction reduces or eliminates the food resources and living space for most species.What is biodiversity?The term biodiversity has to do with the existence of different species that exist in an ecosystem. In a given ecosystem, there could be several species that exist together. This biodiversity is very much important for the continuity of balance in nature.
The actions by humans that decrease biodiversity and threaten sustainability are;
Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions.Pollution from building and commercialization can create health problems in exposed organisms.Habitat destruction reduces or eliminates the food resources and living space for most species.Learn more about biodiversity:https://brainly.com/question/13073382
#SPJ1
Each of the colored lines in hydrogen's emission spectrum corresponds with the
A) energy released by the electrons as they orbit the nucleus.
B) the ionization energy of the electron when forming chemical bonds.
C) transition of an electron from a higher energy level to a lower energy level.
D) transition of an electron from a lower energy level to a higher energy level
when excited.
Answers
Each of the colored lines in hydrogen's emission spectrum corresponds with the C. transition of an electron from a higher energy level to a lower energy level.
What is an emission spectrum?An emission spectrum can be defined as the range of wavelengths, frequencies or pattern of bright lines which can be seen when the electromagnetic radiation that are emitted by an atom of a chemical compound (substance) moves through a spectrometer.
Generally, this process leads to the quantization or transfer of an electron from one energy level to another such as the transition of an electron from a energy level of higher magnitude to a lower energy level in hydrogen's emission spectrum.
Read more on electrons here: https://brainly.com/question/16554158
1. How successful were you in experimentally determining the density of water? How successful were you in experimentally determining the density of ethanol? How do you know? 2. Did the two different ways of finding the density of the metal give the expected result? What is the expected result?
Answers
I experimentally determined the density of water, ethanol, and a metal. The densities of water and ethanol were within the expected range of values, and the two methods of finding the density of the metal gave similar results.
1. I was successful in experimentally determining the density of water and ethanol. I know this because the densities that I calculated were within the expected range of values. The density of water is typically between 0.99 and 1.00 g/cm³, and the density of ethanol is typically between 0.78 and 0.80 g/cm³. The values that I calculated were within these ranges, which indicates that my experimental methods were accurate.
2. The two different ways of finding the density of the metal gave similar results. The expected result is that the two methods would give the same result, since the density of a substance is a constant value. However, there may be some slight differences in the results due to experimental error. For example, the mass of the metal may not have been measured exactly, or the volume of the metal may not have been measured exactly. These small differences in the measurements can lead to small differences in the calculated densities.
Here are the steps that I took to determine the density of water and ethanol:
1. I weighed a graduated cylinder empty.
2. I added water to the graduated cylinder until it reached a certain volume.
3. I weighed the graduated cylinder with the water.
4. I subtracted the weight of the empty graduated cylinder from the weight of the graduated cylinder with the water to get the mass of the water.
5. I divided the mass of the water by the volume of the water to get the density of the water.
I repeated the same steps to determine the density of ethanol.
Here are the steps that I took to determine the density of the metal:
1. I weighed the metal.
2. I placed the metal in a graduated cylinder filled with water.
3. I noted the volume of the water before and after the metal was added.
4. I subtracted the volume of the water before the metal was added from the volume of the water after the metal was added to get the volume of the metal.
5. I divided the mass of the metal by the volume of the metal to get the density of the metal.
To know more about the densities refer here,
https://brainly.com/question/32858407#
#SPJ11
When the dry ingredients of a cake are combined, what is the result?
atom
compound
mixture
element
Answers
When the dry ingredients of a cake are combined, the result is a mixture. Option 3.
What are mixtures?In chemistry, mixtures are substances that are obtained by mixing two or more chemically different substances together with each substance maintaining its chemical identity. In other words, the components of a mixture are still chemically unique and can be separated by physical means.
Mixtures can be homogeneous or heterogeneous. Homogenous mixtures are mixtures in which the components are uniformly dispersed throughout the entire mixture. For heterogeneous mixtures, the components are not uniform throughout the mixture.
Thus, when the dry ingredients of a cake, such as flour, sugar, etc., are combined, what we are going to have is a mixture. Whether this mixture would be homogenous or heterogeneous will depend on the level of mixing. Whatever the case may be, the components can still be separated by physical means.
More on mixtures can be found here: https://brainly.com/question/24898889
#SPJ1
what was the 'elixir of life'
Answers
A 57.6 g sample of methane (CH4) is found to contain 43.2 g of carbon. What is the
percent composition of Carbon and Hydrogen? How much hydrogen (in grams) would a
37.8 g sample of methane contain?
Answers
Answer:
Explanation:
The atomic mass of carbon is 12.011 g/mol.The atomic mass of hydrogen is 1.00794 g/mol.This means that the atomic mass of methane is 12.011+4(1.00794)=16.04276 g/mol.
The percent composition by mass of carbon is \(\frac{12.011}{16.04276} \times 100=\boxed{74.9\%}\)The percent composition by mass of hydrogen is \(\frac{4(1.00794)}{16.04276} \times 100=\boxed{25.1\%}\)This means that in a 37.8-gram sample of methane, there would be \((37.8)\left(\frac{25.1}{100} \right)=\boxed{9.49}\) grams of hydrogen.

What effect does temperature have on reaction rate?
Answers
Answer:
Increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions.
Explanation:
Hope this helps :)
classify the following characteristics and examples of molecules depending on whether they are organic or inorganic.
Answers
Organic compounds;
Carbohydrates lipids and nucleic acidscontained in living organisms Molecules that contain carbon and hydrogen bondsInorganic molecules;
Sometimes contain carbon but do not contain C-H bonds Molecules made of a combination of elements Water and table saltsWhat is an organic molecule?An organic molecule are the molecules that contain long chains of carbon and are studied as part of organic chemistry. Inorganic compounds do not contain catenated carbon chains and are studied as part of inorganic chemistry.
Let us now classify the compounds;
Organic compounds;
Carbohydrates lipids and nucleic acidscontained in living organisms Molecules that contain carbon and hydrogen bondsInorganic molecules;
Sometimes contain carbon but do not contain C-H bonds Molecules made of a combination of elements Water and table saltsLearn more about organic compounds:https://brainly.com/question/5994723
#SPJ1

PLSS HELP I GIVE BRAINLIEST

Answers
Answer:
you r correct it is layer a and b
Explanation:
A student has two samples of NaCl, each one from a different source. Assume that the only potential contaminant in each sample is KCl. The student runs an experiment to determine the percent by mass of chlorine in each sample. From the results of this experiment alone, which of the following questions is most likely to be answered? A. Which sample has the higher purity? B. Which sample has the higher density? C. What is the source of the contaminants present in each of the samples? D. Which sample came from a salt mine, and which sample came from the ocean?
Answers
Answer:
The correct option is;
A. Which sample has the higher purity
Explanation:
The information given relate to the presence of two samples of NaCl, from different sources
The only potential contaminant in each of the sources = KCl
The content of the sample = NaCl
The molar mass of NaCl = 58.44 g/mol
The molar mass of KCl = 74.5513 g/mol
Let the number of moles of KCl in the sample = X
For a given mass of NaCl, KCl mixture, we have;
The molar mass of potassium = 39.0983 g/mol
The molar mass of chlorine = 35.453 g/mol
The molar mass of sodium ≈ 23 g/mol
Therefore;
Each mole of KCl, will yield 35.453 g/mol per 74.5513 g/mol of KCl
While each mole of NaCl will yield 35.453 g/mol per 58.44 g/mol of NaCl
Therefore, the pure sodium chloride sample will yield more chlorine per unit mass of sample.
As such if the two samples have the same mass, the sample with the contaminant of KCl will yield less mass of chlorine per unit mass of the sample, from which the student will be able to tell the purity of the solution.
The sample with the higher purity will yield a higher mass chlorine per unit mass of the sample.
The question that will be most likely answered is ( A ) ; which sample has the higher purity
From the question there are two samples of NaCl from different sources that has a contaminant of KCl in each of them .
A student running an experiment to determine the percentage by mass of Chlorine in each sample is to determine which of the sample contains a lesser amount of the contaminant KCl.
Note : The purer the NaCl sample the more chlorine per unit mass of sample.
Hence the question that can be answered by this experiment is which sample has the higher purity.
Learn more about purity : https://brainly.com/question/18634105
What is the iupac name for the following compound? 3,5-dichloro-4-methylheptanedioic acid
Answers
The IUPAC name for the compound 3,5-dichloro-4-methylheptanedioic acid is 3,5-dichloro-4-methylheptanedioic acid itself. The name provides important information about the structure and composition of the compound.
Explanation:
Let's break down the name to understand its meaning. "3,5-dichloro" indicates that there are chlorine atoms attached to the carbon atoms at positions 3 and 5 of the carbon chain. "4-methyl" indicates that there is a methyl group attached to the carbon atom at position 4.
"Heptanedioic acid" indicates that the compound is an acid and contains a seven-carbon chain with two carboxylic acid groups (-COOH) attached to it.
The numbering of the carbon atoms starts from the carboxylic acid group closest to the main carbon chain. In this case, the carbon atom at position 1 is part of the carboxylic acid group, and the main carbon chain starts from position 2. Therefore, the compound is named as 3,5-dichloro-4-methylheptanedioic acid.
The IUPAC naming system follows specific rules to provide a standardized and unambiguous way to name chemical compounds. The name is based on the structural information of the compound, indicating the positions and types of functional groups, substituents, and the length of the carbon chain. This helps chemists to identify and communicate the exact structure and composition of a compound.
Learn more about IUPAC here :
brainly.com/question/30086566
#SPJ11
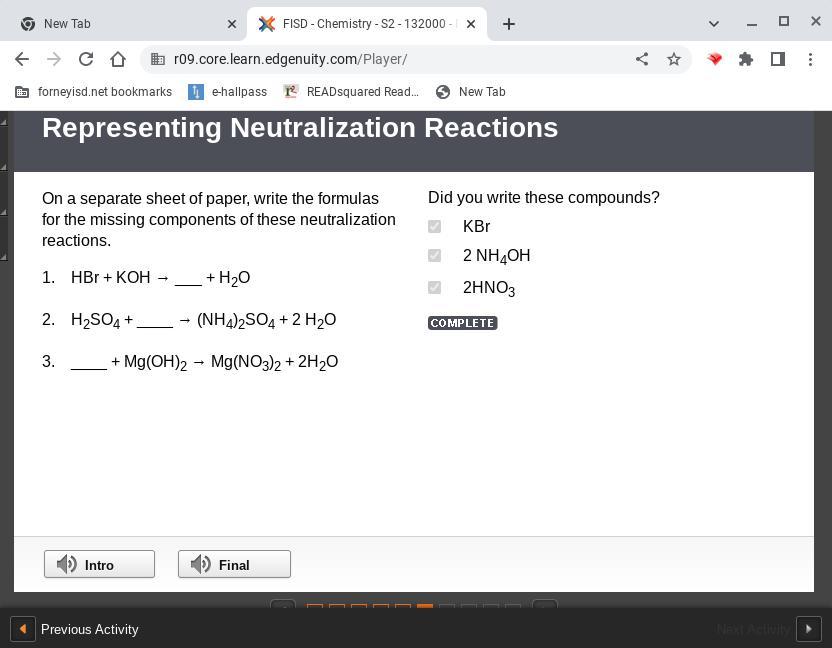
On a separate sheet of paper, write the formulas for the missing components of these neutralization reactions. 1. hbr koh → ___ h2o 2. h2so4 ____ → (nh4)2so4 2 h2o 3. ____ mg(oh)2 → mg(no3)2 2h2o
Answers
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
What is a neutralization reaction?A neutralization reaction is a reaction that occurs between an acid and a base to yield salt and water only.
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
Learn more about neutralization:https://brainly.com/question/15395418
#SPJ4
Answer:
KBr
2 NH4OH
2 HNO3
Explanation:
The decomposition of hydrogen peroxide in the presence of potassium iodide is believed to occur by the following mechanism: step 1 slow: H2O2+I−→H2O+OI− step 2 fast: H2O2+OI−→H2O+O2+I− (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form k[A]m[B]n.., where ' 1′ is understood (so don't write it) for m, n etc.) Rate =
Answers
The overall reaction equation can be obtained by adding the two steps of the mechanism ' 2H\(_{2}\)O\(_{2}\) + 2I- → 2H\(_{2}\)O + I\(_{2}\) '.
The overall reaction equation represents the combined effect of both steps in the mechanism, showing the reactants and products with the smallest integer coefficients.
None of the species act as a catalyst in this mechanism.
In this mechanism, there is no species that acts as a catalyst, meaning there is no substance that speeds up the reaction without being consumed.
OI- acts as a reaction intermediate.
OI- is considered a reaction intermediate because it is formed in one step and consumed in a subsequent step, but it is not present in the overall reaction.
The rate law for the overall reaction can be determined by examining the slow step (step 1) ' Rate = k[H\(_{2}\)O\(_{2}\)][I-] '.
The rate law for the overall reaction is determined by the slow step, which involves the concentration of H\(_{2}\)O\(_{2}\) and I-. The rate is proportional to the concentration of these species, represented by [H\(_{2}\)O\(_{2}\)][I-].
You can learn more about reaction equation at
https://brainly.com/question/11231920
#SPJ11
When is the change of in enthalpy when 77. 2 grams of steam at 100c is converted liquid water at the same temperature and temperature?
Answers
The change in enthalpy, or heat of vaporization, when 77.2 grams of steam at 100°C is converted to liquid water at the same temperature is approximately 40.7 kJ/mol.
This value represents the amount of energy that must be removed from the steam to condense it into liquid water at 100°C. It is important to note that this value may vary slightly depending on the exact pressure and other conditions of the system.
The change in enthalpy, also known as the enthalpy of vaporization, occurs when steam is converted to liquid water at the same temperature. For this process, 77.2 grams of steam at 100°C is converted to liquid water at 100°C.
To calculate the change in enthalpy, we can use the formula:
ΔH = m × ΔHvap
where ΔH is the change in enthalpy, m is the mass of the steam (77.2 grams), and ΔHvap is the enthalpy of vaporization of water (approximately 40.7 kJ/mol at 100°C).
First, we need to convert the mass of steam to moles using the molar mass of water (18.015 g/mol):
moles of steam = (77.2 g) / (18.015 g/mol) ≈ 4.29 moles
Now we can calculate the change in enthalpy:
ΔH = (4.29 moles) × (40.7 kJ/mol) ≈ 174.6 kJ
So, the change in enthalpy when 77.2 grams of steam at 100°C is converted to liquid water at the same temperature is approximately 174.6 kJ.
Visit here to learn more about enthalpy : https://brainly.com/question/29145818
#SPJ11
To ensure that a vehicle crash is inelastic, vehicle safety designers add crumple zones to vehicles. A crumple zone is a part of a vehicle designed to crumple easily in a crash. Use Newton’s second law to explain why crumple zones reduce the force in a collision.
Answers
Answer:
well because with the velocity of the two, using the second law, it can slow the velocity before there is a casualty.
Explanation:
How is enthalpy related to the spontaneity of a reaction?
1. ΔH > 0 contributes to spontaneity.
2. ΔH < 0 contributes to spontaneity.
3. ΔH = 0 contributes to spontaneity.
4. ΔH does not affect spontaneity.
Answers
2. ΔH < 0 contributes to spontaneity, related to the spontaneity of a reaction.
Enthalpy (ΔH) is a measure of the energy of a system and can be used to predict whether a reaction is spontaneous or not. If the enthalpy of a reaction is negative (ΔH < 0), then the reaction is spontaneous. This is because the system is releasing energy, meaning that the reaction is more likely to proceed on its own without any external input. Therefore, a reaction with a negative enthalpy (ΔH < 0) is more likely to be spontaneous than a reaction with a positive enthalpy (ΔH > 0).
learn more about Enthalpy Refer:brainly.com/question/13996238
#SPJ1
9) Which compound is ionic?
a. CO2
b. H2O
c. KCl
d. SCl2
Answers
How old is a piece of cotton cloth if the half-life of carbon-14 is 5,730 years, and the carbon-14 composition of the cloth is 22 percent that of living plants?
Answers
Answer:5600 years
Explanation:
the amount of time it takes for the quantity to lose 50% (Half life) of its value. The half-life of carbon-14 is about 5600 years. Thus, after 5600 years, a piece of cotton cloth will contain half the carbon-14 that was in the original cotton plant.
Answer is 5600 years
How many 4d electrons would be predicted in the ground state for the following elements?a. zirconiumb. cadmiumc. iridiumd. iron
Answers
In order to answer the question first we must write the atomic number of each element:
Zirconium (Zr): 40
Cadmium (Cd): 48
Iridium (Ir): 77
Iron (Fe): 26
Then, we have to complete the distribution of electrons in each orbital for each atom:
The first 4 levels have the following distribution:
Level1: 1s
Number of electrones: 2
Level 2: 2s, 2p
Number of electrones 8 (2 in the s orbital and 6 in the p orbitals).
Level3: 3s, 3p, 3d
Number of electrones 18 (2 in the s orbital, 6 in the p orbital and 10 in the d orbitals)
Level 4: 4s, 4p, 4d, 4f
Number of electrones 32 (2 in the s orbital, 6 in the p orbitals, 10 in the d orbitals and 14 in the f orbitals)
The order in which the orbitlas are completed depends on the energy of each level. For example the 4s orbitals will be completed before the 3d orbitals because their energy is lower.
The order is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p...
Now, knowing the atomic number we can answer the question:
For Zirconium (total 40 electrones):
\(1s^2,2s^2,2p^6,3s^2,3p^6,4s^2,3d^{10},4p^6,5s^2,4d^2\)2 electrones are predicted in the 4d orbital
For Cadmium (total 48 electrones):
\(1s^2,2s^2,2p^6,3s^2,3p^6,4s^2,3d^{10},4p^6,5s^2,4d^{10}^{}\)10 electrones are predicted in the 4d orbital
For iridium, as it has an atomic number higher than Cadmium we can predict tha it also complets the 4d orbital, then it has also 10 electrones in it.
For iron (total 26 electrones)
\(1s^2,2s^2,2p^6,3s^2,3p^64s^2,3d^6\)Iron has no electrones in the 4d orbitals
A car starts from rest and reaches a top speed of 80 m/s. If the car did this is 20 seconds . What is the acceleration ?
Answers
somethingExplanation:
The density of a gaseous organic compound is 340g/L at 45°C and 1.7atm. what is it's mole
Answers
To determine the number of moles of the gaseous organic compound, we can use the ideal gas law equation: PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
How to calculate ?First, we need to convert the density to mass per volume. The density of the gas is given as 340g/L. Therefore, the mass of 1 L of the gas is 340 g.
Next, we need to use the ideal gas law to calculate the number of moles. We know that the pressure is 1.7 atm, the temperature is 45°C (which is 318 K), and the volume can be calculated using the density and the molar mass of the compound. The molar mass can be determined from the molecular formula of the compound.
Assuming the compound is a hydrocarbon, we can use an average molar mass of 28. Thus, the volume of 1 mole of the gas can be calculated as follows:
V = (molar mass/density) × 1000 ml/L = (28/340) × 1000 = 82.35 ml/mol
Using the ideal gas law equation and plugging in the given values, we get:
n = (PV) / (RT) = (1.7 atm × 82.35 ml) / (0.0821 L atm/mol K ×318 K) = 0.839 mol
Therefore, the number of moles of the gaseous organic compound is 0.839 mol
To know more about Moles and Molar mass ,visit :
https://brainly.com/question/12007096
#SPJ9
how to tell the difference between ionic and covalent bonds
Answers
Comparing the electronegativities of the two elements is one method of predicting the type of bond that will form between them.
Ionic bonds are produced between atoms of metals and non-metals where the metal loses an electron to complete its octet and the non-metal acquires that electron to complete its octet. Covalent bonds are formed when two atoms share electrons to complete their octets.
Ionic chemicals are bound together by ionic bonds, whereas covalent compounds are held together by strong covalent bonds. While covalent molecules are normally insoluble in water, ionic compounds are. Additionally, covalent molecules are typically more flammable than ionic ones.
If the electronegativity of the two atoms differs by enough to allow one to totally draw an electron away from the other, the connection is ionic.
To know more about covalent bond:
https://brainly.com/question/32676803
#SPJ4
How many grams of potassium chloride are produced if 25g of potassium chlorate decompose.
Answers
Answer:
2.05
Explanation:
Answer:15 g KCl
Explanation: 2KClO3->2KCI+3O2
A 2.8 g sample of pure metal requires 10.1 J of energy to change its temperature from 21°C to 36°C. What is this metal? Substance Specific Heat Gold 0.129 J/g °C Silver 0.237 J/g °C Copper 0.385 J/g °C Water 4.18 J/g °C
Answers
Answer:
Silver 0.237 J/g °C
Explanation:
We know that the heat required to raise the temperature of a given mass of a substance through a given temperature rise is given by;
H = mcθ
Where;
m = mass of the body
c = specific heat capacity of the body
θ = temperature rise
10.1 J = 2.8 g * c * (36°C - 21°C)
c = 10.1 J / 2.8 g * (36°C - 21°C)
c = 0.24 J/g °C
(science)
if you have visited a natural history museum, you have probably seen the dinosaur exhibits. of course, dinosaurs do not exist today. we know about them because of the discovery of what?
a. herds
b. fossils
c. stories
d. hatchlings
Answers
b. fossils we know about them because of the discovery .
What proof does science have that dinosaurs ever lived on Earth?The Earth was the home of the dinosaurs for at least 230 million years, according to a wealth of fossilized bones, teeth, trackways, and other physical evidence. But no dinosaur bones have been discovered in strata younger than 66 million years old, not even a single trace.
In June 2022, Dippy returned to the Natural History Museum as a component of a traveling exhibition. The replica will then be loaned to another institution for a period of three years. Applications for the future location were made available by the Museum in March 2022.
Learn more about fossils refer
https://brainly.com/question/19083813
#SPJ4
A 500.0 g block of dry ice (solid CO₂, molar mass = 44.0 g) vaporizes to a gas at
room temperature. Calculate the volume of gas produced at 25.0 °C and 1.75
atm.
Show your work
Answers
When solid carbon dioxide (dry ice) vaporizes to gas, it undergoes a phase change from solid to gas without melting into a liquid. This process is called sublimation.
To calculate the volume of gas produced, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
First, we need to determine the number of moles of gas produced. We can use the molar mass of carbon dioxide to convert from mass to moles:
moles of CO₂ = mass of dry ice / molar mass of CO₂
moles of CO₂ = 500.0 g / 44.0 g/mol
moles of CO₂ = 11.36 mol
Since the dry ice sublimes directly to a gas, all of the moles of CO₂ will be in the gas phase.
Next, we can plug in the values we know into the ideal gas law:
PV = nRT
V = nRT / P
where R is the ideal gas constant, which has a value of 0.08206 L·atm/(mol·K).
Converting the temperature to Kelvin:
T = 25.0 °C + 273.15 = 298.15 K
Plugging in the values:
V = (11.36 mol) x (0.08206 L·atm/(mol·K)) x (298.15 K) / (1.75 atm)
V = 439.4 L
Therefore, the volume of gas produced is approximately 439.4 L.
When solid carbon dioxide (dry ice) vaporizes to gas, it undergoes a phase change from solid to gas without melting into a liquid. This process is called sublimation.
To calculate the volume of gas produced, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
First, we need to determine the number of moles of gas produced. We can use the molar mass of carbon dioxide to convert from mass to moles:
moles of CO₂ = mass of dry ice / molar mass of CO₂
moles of CO₂ = 500.0 g / 44.0 g/mol
moles of CO₂ = 11.36 mol
Since the dry ice sublimes directly to a gas, all of the moles of CO₂ will be in the gas phase.
Next, we can plug in the values we know into the ideal gas law:
PV = nRT
V = nRT / P
where R is the ideal gas constant, which has a value of 0.08206 L·atm/(mol·K).
Converting the temperature to Kelvin:
T = 25.0 °C + 273.15 = 298.15 K
Plugging in the values:
V = (11.36 mol) x (0.08206 L·atm/(mol·K)) x (298.15 K) / (1.75 atm)
V = 439.4 L
Therefore, the volume of gas produced is approximately 439.4 L.
Learn more about temperature on:
https://brainly.com/question/7510619
#SPJ6
What is the relationship between specific heat and the rate of heating?
Answers
Answer:
the rate of heat transfer is directly proportional to the mass flow rate.
Explanation:
There is an elementary equation from basic thermodynamics that states that the rate of heat transfer (Q) equals the mass flow rate (M) times a Constant (the specific heat of water) times the Delta T (fluid temp out minus fluid temp in): Q = M x C x Delta T In other words, the rate of heat transfer is directly proportional to the mass flow rate.
as atomic radius decreases, both ionization energy and electronegativity a increases b decrease c stay the same
Answers
Answer: B!!!
Explanation:
Balance the Following Equations: SiCl4 + H2O = SiO2 + HCl
A) 1 SiCl4 + 1 H2O = 2 SiO2+ 4 HCl
B) 2 SiCl4 + 2 H2O = 1 SiO2+ 4 HCl
C) 2 SiCl4 + 1 H2O = 1 SiO2+ 4 HCl
D) 1 SiCl4 + 2 H2O = 1 SiO2+ 4 HCl
Answers
SiCl4 + 2H2O → SiO2 + 4HCl (balanced equation)
a proton (q = 1.6x10-19 c) moves along a uniform e-field (3.0 v/m) for a distance of 10 cm. what is the change in kinetic energy of the proton?
Answers
A proton (q = 1.6x10-19 c) travels 10 cm along a constant e-field (3.0 v/m). The proton has changed its kinetic energy by 4.8 x 10-20 J after travelling 10 cm in the electric field.
The equation: may be used to determine the change in kinetic energy of a charged particle travelling in an electric field. K = qEd, where q is the particle's charge (1.6 x 10-19 C), E is the intensity of the electric field (3.0 V/m), and d is the distance travelled (10 cm).
Since the electric field is expressed in volts per metre, we must first convert the distance from centimetres to metres. 10 cm = 0.10 m.
After that, we can enter the values into the equation and find K:
K = qEd = 4.8 x 10-20 J = (1.6 x 10-19 C)(3.0 V/m)(0.10 m)
The proton's kinetic energy changes by 4.8 × 10-20 J after travelling 10 cm in the electric field.
learn more about kinetic energy here:
https://brainly.com/question/26472013
#SPJ4