The statements in the table summarize what Johannes Kepler stated in the early 1600s about the motion of planets.
Kepler's Statements
The orbit of a planet around the sun is an ellipse.
As a planet orbits the sun, a line between the planet and the sun sweeps out equal areas during equal periods of time.
There is a mathematical relationship between the time it takes a planet to orbit the sun and the average distance the
planet is from the sun.
Over the last four hundred years, predictions based on his statements have proven true. Which best describes
Kepler's statements?
O hypothesis
O theory
law
explanation
Answers
Answer:
it's law
Explanation:
i got it wrong because i put the wrong answer but it was law or c
i hope this help's
Answer: ITS C
Explanation: Its C Laws
Related Questions
Plzzzz answer quick
An excited electron of hydrogen emits the least energy when it drops from 1 point
quantum levels *
Level 4 to level 2
level 5 to level 1
level 2 to level 1
O level 2 to level 5
Answers
Answer:
sorry to do this but I really needed some points to ask my question from someone else so that's why I am doing so really sorry and thank you for free points
The number of atoms of any element in the given chemical formula is the number that is written on the foot of the symbol of that element. Therefore, the correct option is option C.
What is atom?Atom is the smallest particle of any matter. Atom combines to form element and element combine to form molecule or compound.
Atom consists of electron, proton and neutron. The total mass of atom is inside the nucleus. Inside the nucleus proton and neutron is there. So calculate mass of an atom, total mass of all protons is added to the total mass of neutron. Electrons revolve around the nucleus.
If an electron of hydrogen gets excited and drops from energy level 2 to level 1 to release least energy. The level shows the energy levels.
Therefore, the correct option is option C.
Learn more about atoms, here:
https://brainly.com/question/13518322
#SPJ2
please if anyone knows the answer solve it on the paper with DATA, SOLUTION and RESULT then attach the photo here
The question is:
Calculate the molecular mass (a.m.u) of C2H5OH.
I'll be very thankful to you
Answers
The compound is ethanol
Animal fats and vegetable oils are triacylglycerols, or triesters, formed from the reaction
of glycerol (1, 2, 3-propanetriol) with three long-chain fatty acids. One of the methods
used to characterize a fat or an oil is a determination of its saponification number. When
treated with boiling aqueous KOH, an ester is saponified into the parent alcohol and fatty
acids (as carboxylate ions). The saponification number is the number of milligrams of
KOH required to saponify 1.000 g of the fat or oil. In a typical analysis, a 2.085-g sample
of butter is added to 25.00 mL of 0.5131 M KOH. After saponification is complete, the
excess KOH is back titrated with 10.26 mL of 0.5000 M HCl. What is the saponification
number for this sample of butter?
Answers
Saponification number = (V × M × F × 56.1) / W
Where:
V = volume of HCl used in the back titration
M = molarity of HCl
F = factor of KOH (which is 1 for pure KOH)
W = weight of the butter sample used in grams
First, we need to calculate the amount of KOH used in the saponification reaction:
0.5131 M KOH = 0.5131 moles KOH / liter
25.00 mL KOH = 0.02500 L KOH
moles KOH used = 0.5131 moles/L × 0.02500 L = 0.0128 moles KOH
Since the saponification reaction is a 1:1 reaction between KOH and the triacylglycerol in the butter sample, the amount of butter used is also 0.0128 moles.
Next, we need to calculate the amount of HCl that reacted with the excess KOH:
0.5000 M HCl = 0.5000 moles HCl / liter
10.26 mL HCl = 0.01026 L HCl
moles HCl used = 0.5000 moles/L × 0.01026 L = 0.00513 moles HCl
Since the reaction between HCl and KOH is also a 1:1 reaction, the moles of KOH that were not used in the saponification reaction is equal to the moles of HCl used in the back titration:
moles KOH not used = moles HCl used = 0.00513 moles HCl
To find the saponification number,
Saponification number = (V × M × F × 56.1) / W
Saponification number = (0.01026 L × 0.5000 moles/L × 1 × 56.1) / 2.085 g
Saponification number = 6.50
Therefore, the saponification number for this sample of butter is 6.50.
To know more about Saponification:
https://brainly.com/question/2263502
#SPJ1
Imagine running one hour straight west at 5 km/h and then changing direction quickly and running
one hour straight north at 5 km/h. What was your total displacement? Round to the nearest whole
number.
O 10 km
O 5 km
O 50 km NW
O 7 km NW
Answers
Answer:
If you ran one hour straight west at 5 km/h and then changed direction quickly and ran one hour straight north at 5 km/h, your total displacement would be 7 km NW.
Explanation:
This is because your total change in position would be 5 km to the west and 5 km to the north, resulting in a displacement of 7 km NW.
After running one hour straight west at 5 km/h and then changing direction quickly and running one hour straight north at 5 km/h, the total displacement would be 7 km NW, rounded to the nearest whole number.
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The molarity of the solution prepared by dissolving 0.355 moles of NH₃ in enough water to make 3.84 L of solution is 0.092 M
How do i determine the molarity of the solution?First, we shall list out the given parameters from the question. This is given below:
Number of mole of NH₃ = 0.355 moleVolume of solution = 3.84 LMolarity of solution = ?Molarity of a solution is defined as mole per unit volume i.e
Molarity of solution = mole / volume
Inputting the various parameters, we have:
Molarity of solution = 0.355 / 3.84
Molarity of solution = 0.092 M
Thus, from the above calculation, it is evident that the molarity of the solution is 0.092 M
Learn more about molarity:
https://brainly.com/question/16073358
#SPJ1
Explain what is a chemical reaction.
Answers
Answer:
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Explanation:
Answer:
a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Explanation:
Here's an example. When you burn wood, It releases Carbon dioxide, water vapor, and ashes. It just depends what chemicals you use ^^
How much heat (kJ) is absorbed by 825.4 g of water in order for the temperature to increase from 25.00°C to 32.50°C?
Answers
The heat absorbed by the water is 25.9 KJ
To solve the question given above, we'll begin by calculating the change in the temperature of the water. This can be obtained as follow:
Initial temperature (T₁) = 25 °C
Final temperature (T₂) = 32.50 °C
Change in temperature (ΔT) =? ΔT = T₂ – T₁ΔT = 32.50 – 25
ΔT = 7.5 °CFinally, we shall determine the heat absorbed by the water. This can be obtained as follow:
Mass of water = 825.4 g
Change in temperature (ΔT) = 7.5 °C
Specific heat capacity of water = 4.184 J/gºC
Heat absorbed (Q) =? Q = MCΔTQ = 825.4 × 4.184 × 7.5
Q = 25901.052 J
Divide by 1000 to express in KJ
Q = 25901.052 / 1000
Q = 25.9 KJTherefore, the heat absorbed by the water is 25.9 KJ.
Learn more: https://brainly.com/question/21627758
Determine whether the disruption of the bonds or attractions occurs during protein hydrolysis or protein denaturation.
Answers
Answer:
The disruption of the bonds or attractions occurs during protein hydrolysis which results in the loss for the primacy structure. The peptide bonds is the bond affected in this scenario.
The disruption of the bonds however only exist in the process of denaturation and this results in a change in the confirmation which could be secondary, tertiary, and quaternary structural related. And example of the bonds affected include salt bridges, disulfide bridges, hydrogen bonds etc.
Which factor causes chemical weathering
A gravity
B sunlight
C Acids
D wind
Answers
Answer:
C
Explanation:
because its the answer
Answer:
acids
Explanation:
acids because if the rain comes from cloud is acid it can be weathered or if the water is slightly acidic
ASAP PLEASE!!!B. Complete the drawing for the sample reaction below to show the law of conservation of
mass, when XY is produced.
+
->
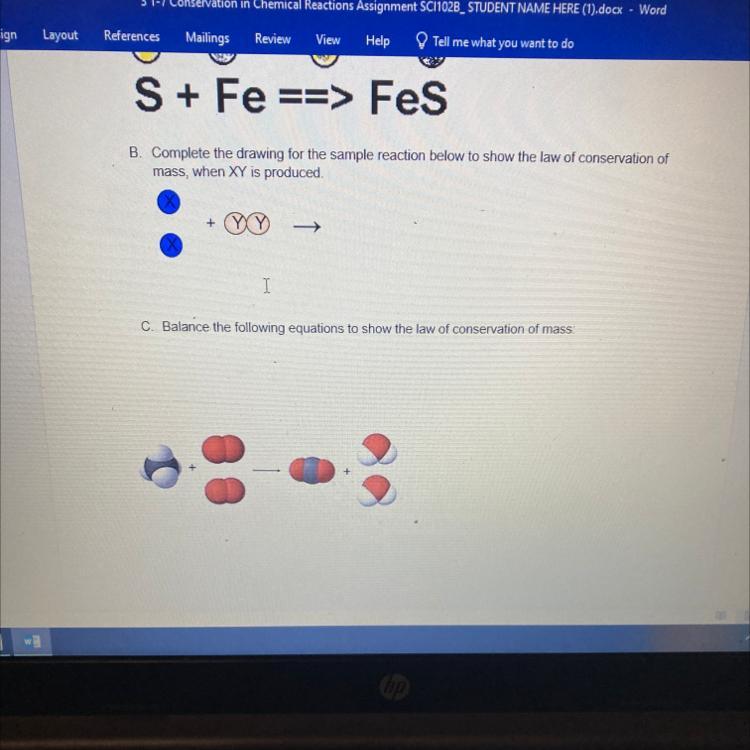
Answers
The complete reaction, according to the law of conservation of mass is:
XX + YY → 2XY
The Law of Conservation is a fundamental principle in chemistry and physics. It states that in a closed system, mass cannot be created or destroyed during a chemical reaction or a physical change. The total mass of the substances involved before the reaction or change must equal the total mass of the substances after the reaction or change.
This principle is based on the understanding that atoms are not created or destroyed, but they can combine or separate to form different substances.
Learn more about the law of mass conservation, here:
https://brainly.com/question/28711001
#SPJ1
WOULD YOU CONSIDER WATER AND OZONE TO BE MOLECULES ?
Answers
Answer:
yes
Explanation:
Water contains molecular bonds and because it is made from more than one element and they are oxygen and hydrogen.
ozone would be considered a molecule because it has three oxygen atoms formed together and is 21% of the gases found on earth
The total pressure of a mixture of CO2 and O2 is 1.03 atm. If the pressure of the CO2 is 560. torr, what's the pressure of O2 in mmHg?
Answers
The pressure (in mmHg) of O₂, given that CO₂ has a pressure of 560 torr is 222.8 mmHg
We'll begin by converting 1.03 atm to torr. This can be obtained as follow:
1 atm = 760 torr
Therefore,
1.03 atm = (1.03 atm × 760 torr) / 1 atm
1.03 atm = 782.8 torr
Next, we shall determine the pressure of O₂. This can e obtained as follow:
Total pressure = 782.8 torr Pressure of CO₂ = 560 torrpressure of O₂ =?Total pressure = pressure of CO₂ + pressure of O₂
782.8 = 560 + pressure of O₂
Collect like terms
Pressure of O₂ = 782.8 - 560
Pressure of O₂ = 222.8 torr
Finally, we shall convert 222.8 torr to mmHg. This is illustrated below
Recall
760 torr = 760 mmHg
Therefore,
222.8 torr = 222.8 mmHg
Thus, we can conclude that the pressure of O₂ is 222.8 mmHg
Learn more about conversion:
https://brainly.com/question/14847010
#SPJ1
Choose any metal atom from Group 1A, 2A, or 3A. What charge will it adopt when it ionizes?
Answers
Answer:
Positive charge.
Explanation:
Hello!
In this case, since elements belonging to groups 1A, 2A and 3A are mostly metals, we infer they have the capacity to lose electrons and therefore they become positively charged.
Some examples may be:
\(Na^+\\\\H^+\\\\Ca^{2+}\\\\Al^{3+}\\\\Sr^{2+}\)
Best regards!
Chlorine gas reacts with fluorine gas to form chlorine trifluoride. Cl2(g)+3F2(g)→2ClF3(g) A 2.05 L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmHg and fluorine gas at a partial pressure of 730 mmHg .
Answers
Answer:
2.4 grams of ClF3
Explanation:
First let us determine the moles of Cl2 and F2,
Cl2 = ( ( 337 )( 2.05 L ) / ( 0.082 )( 298 K ) ) * ( 1 atm / 780 ),
Cl2 = ( 690 / 24.436 ) * ( 1 / 780 ),
Cl2 = ( About ) 0.036 moles of Cl2
_________________________________________________
F2 = ( ( 729 )( 2 L ) / ( 0.082 )( 298 K ) ) * ( 1 atm / 780 ),
F2 = ( 1458 / 24.436 ) * ( 1 / 780 )
F2 = ( About ) 0.078 moles of F2
Now let us identify the limiting reactant, considering the ratio between ClF3 and Cl2 / F2. In this case F2 is the limiting reactant, as it forms a smaller molar ratio;
The theoretic yield is thus performed with the limiting reactant F2,
0.078 * ( 2 / 3 ) * ( 92.45 / 2 ) = ( About ) 2.4 grams of ClF3
Which statement BEST explains why liquids and gases can flow but solids cannot?
A) Liquids and gases usually occupy a larger volume than the solid form of a substance.
B) The structure of liquids and gases is not fixed, so particles can slide past each other.
C) Solid particles are often larger than liquid and gas ones, so they can’t move as easily.
D) Liquid and gas particles are more attracted to each other and “pull” the other particles.
Answers
Answer:
B
Explanation:
A is not the answer. Although the statement is accurate in regards to gases, it does not explain why liquids and gases can flow.
B is the answer. Solids are in fixed structures. When you apply heat or pressure, these structures are broken apart and allowed to move freely.
C is not the answer. This is inaccurate. Changing the phase of a substance does not change the size of the particle.
D is not the answer. The opposite of this statement is true. The attractive forces between particles in a solid allow the substance to hold its structure. When you apply heat or pressure, the attractive forces are overpowered and the structure is broken.
Answer:
B) The structure of liquids and gases is not fixed, so particles can slide past each other.
Explanation:
they are correct took the test
Which statement describes the appearance of a temperature-vs.-time graph?
A horizontal line shows that the temperature increases at a constant rate over time.
A vertical line shows that the temperature decreases at a constant rate over time.
Horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases.
Horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
pls hurry am timed
Answers
The statement that describes the appearance of a temperature-vs.-time graph : c)Horizontal lines where temperature is constant during phase changes connect upward-sloping lines where temperature increases.
What is meant by temperature-vs.-time graph?As the temperature is constant during phase change (melting or boiling), so the graph is a horizontal line. A temperature-energy graph shows energy and temperature changes as water turns from solid, ice, to a liquid, water, to a gas, water vapor.
Temperature increases when a single phase (solid, liquid, or gas) is heated, so graph for these changes has upward slope.
A is wrong as horizontal line shows a constant temperature. B is wrong as there are no vertical lines in heating curve. D is not correct as horizontal line does correspond to an increase in temperature.
To know more about temperature-vs.-time graph, refer
https://brainly.com/question/25100063
#SPJ1
Consider the following information.
A+ KOH → B+ 2 KNO3What is the molecular formula of A, what is the molecular formula for B? You have to balance the A + KOH part of the equation. MM of B = 62.068g/mol
B is 38.67 % C, 9.67% H and 51.56 % O
Answers
Answer:
The molecular formula for A is C2H4(NO3)2
The molecular formula for B is C2H6O2
Explanation:
I linked an image that will show the work.

In which type of rock would scientists most commonly find a fossil of triceratops?
Answers
Answer:
shale and siltstone
Explanation:
The Triceratops dinosaur fossils are approximately 70 million years old, because they are found in shale and siltstone that contain volcanic ash radiometrically dated at 70 million years.
Matter are anything that is made up of atoms. The quantity of matter can be observed only on the basis of mass and volume calculation. Therefore, in shale and siltstone, Scientists would most commonly find a fossil of triceratops.
What is matter?Matter is a substance that has some mass and can occupy some volume. The matter is mainly used in science. Matter can be solid, liquid or gas.
Matter is anything that is made up of atoms. Anything around us that can be physically seen and touched are matter. Ice, water and water vapors are example of matter.
So as we saw that matter has some mass so mass can be measured in gram only. Mass can also be represented as number of molecules. We also saw that matter occupy some volume and that volume is measured only in liter.
Scientists would most commonly find a fossil of triceratops in shale and siltstone that contain volcanic ash radiometrically dated at 70 million years.
Therefore, in shale and siltstone, Scientists would most commonly find a fossil of triceratops.
To learn more about matter, here:
https://brainly.com/question/4562319
#SPJ2
Given 0.08 of KMnO4, calculate the number of molecules
Answers
The number of molecules in the permanganate is 4.8 * 10^22 molecules
What is the number of the molecules?We know that if we are to obtain the number of molecules form the number of the moles of the substances then as a matter of necessity we would have to turn to the Avogadro's law and that is what we are going to do here.
We have that;
If 1 mole contains about 6.02 * 10^23 molecules
0.08 moles would contain 0.08 * 6.02 * 10^23/ 1
= 4.8 * 10^22 molecules
Hence, we have about 4.8 * 10^22 molecules in the permanganate
Learn more about molecules:https://brainly.com/question/19922822
#SPJ1
Which part of the stem transports water to the rest of the plant? A.root cells b. xylem c.bark d. phloem
Answers
Answer:
B
Explanation:
Answer:
xylem
Explanation:
Look at Table 4 in the procedure portion of the experiment. Calculate the pH you would expect each of the buffer solutions (A, B, C, D, and E) to be using the Henderson-Hasselbalch equation, assuming that the solutions of acetic acid and sodium acetate are equimolar.
Answers
The pH of the buffer solutions as determined using the Henderson–Hasselbalch equation are:
A. pH = 4.75B. pH = 4.05C. pH = 3.75D. pH = 5.75E. pH = 5.45What is the pH of the solutions?The pH of a buffer is determined using the Henderson–Hasselbalch equation shown below:
pH = pKₐ + log([A⁻]/[HA])A. Volume of acetic acid = 5 mL; Volume of sodium acetate = 5 mL; pka of acetic acid = 4.75
The solutions of acetic acid and sodium acetate are equimolar;
pH = 4.75 + log(1)
pH = 4.75
B. Volume of acetic acid = 5 ml; Volume of sodium acetate = 1 mL; pka of acetic acid = 4.75
The solutions of acetic acid and sodium acetate are equimolar;
pH = 4.75 + log(1/5)
pH = 4.05
C. Volume of acetic acid = 10 ml; Volume of sodium acetate = 1 mL; pka of acetic acid = 4.75
The solutions of acetic acid and sodium acetate are equimolar;
pH = 4.75 + log(1/10)
pH = 3.75
D. Volume of acetic acid = 1 ml; Volume of sodium acetate = 10 mL; pka of acetic acid = 4.75
The solutions of acetic acid and sodium acetate are equimolar;
pH = 4.75 + log(10/1)
pH = 5.75
E. Volume of acetic acid = 1 ml; Volume of sodium acetate = 5 mL; pka of acetic acid = 4.75
The solutions of acetic acid and sodium acetate are equimolar;
pH = 4.75 + log(5/1)
pH = 5.45
In conclusion, the pH of the buffer solutions are determined using the Henderson–Hasselbalch equation.
Learn more about buffers at: https://brainly.com/question/22390063
#SPJ1

98.96g/mol of CH2O what will be the chemical formula
Answers
Let's break down the molar mass of CH2O:
- Carbon (C) has a molar mass of approximately 12.01 g/mol.
- Hydrogen (H) has a molar mass of approximately 1.01 g/mol.
- Oxygen (O) has a molar mass of approximately 16.00 g/mol.
Now, let's calculate the molar mass of CH2O:
(1 x molar mass of C) + (2 x molar mass of H) + (1 x molar mass of O)
= (1 x 12.01 g/mol) + (2 x 1.01 g/mol) + (1 x 16.00 g/mol)
= 12.01 g/mol + 2.02 g/mol + 16.00 g/mol
= 30.03 g/mol
The molar mass of CH2O is approximately 30.03 g/mol, which is different from the given molar mass of 98.96 g/mol.
It seems that there might be an error or misunderstanding in the given molar mass value. The correct chemical formula for a compound with a molar mass of 98.96 g/mol cannot be determined based on the information provided.
What do we call living things that share characteristics including processes that make life possible?
Question 3 options:
chemical properties
structure
organisms
energy
Answers
Organisms
Step-by-step explanation:
Properties of Life. All living organisms share several key characteristics or functions: order, sensitivity or response to the environment, reproduction, growth and development, regulation, homeostasis, and energy processing.
738.90 m has ____ significant figures
Answers
Answer: 4
Explanation: because the zero doesn't count
When comparing a prokaryotic cell to a eukaryotic cell, an important difference is that the prokaryotic cell-
is simple, performing limited functions.
is relatively small in size and unorganized.
has its genetic information stored in the nucleus.
has no structures that allow it to store food.
Answers
a graduated cylinder to find the volume of the cell.
a triple-beam balance to find the mass of the cell.
a microscope to determine if the cell has a nucleus.
What is the difference between fat-soluble and water-soluble vitamins? Name two examples of each type?
Answers
Which statements true about the total mass of the reactants during a chemical change? (5 points)
0
his destroyed during chemical reaction,
It is less than the total mass of the produce
It is equal to the total mass of the products,
is greater than the total mass of the products
Answers
HELP PLS, ILL GIVE BRAINLIEST, 100 POINTSAbove is the word equation for photosynthesis. Respiration is another biochemical process that takes place within animals. The molecules involved in photosynthesis and respiration are water (H2O), carbon dioxide (CO2), oxygen (O2) and glucose (C6H12O6).
Select the equation below that best represent the process of photosynthesis.
Select one:
A.
B.
C.
D.

Answers
Answer:
C
Explanation:
Answer:
A.)
Explanation:
Can someone help me?

Answers
The new volume assuming that the pressure and temperature remain constant is 0.46 L and the correct option is option 1.
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as-
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
Given,
Initial volume = 1.5 L
Initial moles = 7.5 moles.
Moles remaining = 2.3 moles
\(\frac{n_{1} }{V_{1} } = \frac{n_{2} }{V_{2} }\)
\(\frac{7.5}{1.5 } = \frac{2.3}{V_{2} } }\)
V₂ = 0.46 L
Thus, the ideal selection is option 1.
Learn more about Ideal Gas Law, here:
https://brainly.com/question/12624936
#SPJ1
A 20 g granite boulder absorbs 300.2 Joules of energy from the Sun, resulting in its temperature
changing. Calculate this temperature change
Answers
Answer:
19 °C
Explanation:
Step 1: Given and required data
Mass of granite (m): 20 gHeat absorbed (Q): 300. 2 JSpecific heat capacity of granite (c): 0.790 J/g.°CStep 2: Calculate the temperature change (ΔT)
We will use the following expression.
Q = c × m × ΔT
ΔT = Q/c × m
ΔT = 300.2 J/(0.790 J/g.°C) × 20 g = 19 °C