name the four physical properties of metals
Answers
Answer:
Metal Physical Properties:
Lustrous (shiny)
Good conductors of heat and electricity.
High melting point.
High density (heavy for their size)
Malleable (can be hammered)
Ductile (can be drawn into wires)
Usually solid at room temperature (an exception is mercury)
Related Questions
Calculate the work done if:
F= 160 N and d = 0.1 m
Answers
Answer: 16 Nm
Explanation: Work is measured as Force times Displacement . Therefore , 160N X 0.1m = 16 Nm
Use the following information to answer the following question.
Cu + 2 AgNO3 --> Cu(NO3)2 + 2 Ag
12.7 g of copper metal reacts with silver nitrate to actually produce 38.1 g of silver in the lab.
Percent Yield = Actual Yield/Theoretical Yield x 100%
What is the percent yield of silver (Ag) in this reaction?
Answers
Answer:
For these problems, we need to compare the theoretical yield that we'd get from performing stoichiometry to the actual yield stated in the problem. % yield is the actual yield/theoretical yield x 100%
Cu + 2 AgNO₠→ Cu(NOâ‚)â‚‚ + 2 Ag ==> each mole of copper yields two moles of silver
12.7-g Cu x ( 1 mol Cu /63.5-g Cu) x ( 2 mol Ag / 1 mol Cu) x (108-g Ag / 1 mol Ag) = 43.2-g Ag. This is the theoretical yield. Now, since we got 38.1-g Ag our % yield is:
38.1-g/43.2-g x 100% = 88.2%
Explanation:
The reversible reaction between hydrogen chloride gas and one mole of oxygen gas produces steam and chlorine gas. Predict the direction in which the system will move to reach equilibrium if one starts with:
(a) P(h2o) = P(hcl) = p(o2) = 0.2 atm
(b) P(hcl) = 0.3 atm, P(h2o) = 0.35 atm, P(cl2) = 0.2 atm, and P(o2) = 0.15 atm
I don't get how to set up my calculations...
Answers
(a) P(H2O) = P(HCl) = P(O2) = 0.2 atm The reaction is balanced, and there is no net tendency for the system to shift in either direction.
(b) P(HCl) = 0.3 atm, P(H2O) = 0.35 atm, P(Cl2) = 0.2 atm, and P(O2) = 0.15 atm
If Q < K, the system will shift to the right (forward reaction).
If Q > K, the system will shift to the left (reverse reaction).
If Q = K, the system is already at equilibrium.
To predict the direction in which the system will move to reach equilibrium, we need to compare the initial pressures with the equilibrium expression for the reaction:
2HCl(g) + O2(g) ⇌ 2H2O(g) + Cl2(g)
The equilibrium expression for this reaction is given by:
K = \([H2O]^2[Cl2] / [HCl]^2[O2]\)Now let's analyze each case:
(a) P(H2O) = P(HCl) = P(O2) = 0.2 atm
Since the initial pressures of all the species are equal, we can say that the system is already at equilibrium. The reaction is balanced, and there is no net tendency for the system to shift in either direction.
(b) P(HCl) = 0.3 atm, P(H2O) = 0.35 atm, P(Cl2) = 0.2 atm, and P(O2) = 0.15 atm. To determine the direction of the system's shift, we need to compare the calculated Q (reaction quotient) with the equilibrium constant (K).
Q =\([H2O]^2[Cl2] / [HCl]^2[O2]\)
Q = \((0.35)^2(0.2) / (0.3)^2(0.15)\)
Now compare Q to K:
If Q < K, the system will shift to the right (forward reaction).
If Q > K, the system will shift to the left (reverse reaction).
If Q = K, the system is already at equilibrium.
For more such questions on reaction
https://brainly.com/question/24795637
#SPJ11
what is the net cell reaction for the chromium-silver voltaic cell?
Answers
Cr(s) → Cr3+(aq) + 3e-Ag+(aq) + e- → Ag(s)
To solve such this we must know the concept of chemical reaction. Therefore, the balanced net cell reaction for the chromium-silver voltaic cell is Cr(s)+3Ag⁺(aq)→Cr³⁺(aq)+3Ag(s)
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction. Chromium-silver voltaic is a cell that is used in electrochemistry.
The balanced net cell reaction for the chromium-silver voltaic cell is
Cr(s)+3Ag⁺(aq)→Cr³⁺(aq)+3Ag(s)
Therefore, the balanced net cell reaction for the chromium-silver voltaic cell is
Cr(s)+3Ag⁺(aq)→Cr³⁺(aq)+3Ag(s)
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ2
the condensed formula ch3ch(ch3)2 represents
Answers
The molecular formula CH₃CH(CH₃)₂ is the condensed formula for the hydrocarbon methylpropane.
What are organic compounds?Organic compounds are compounds that are obtained from organic materials.
The most common organic compound are the hydrocarbons,
The hydrocarbons includes the alkanes, alkenes, alkynes, etc.
The given compound CH₃CH(CH₃)₂ is the condensed formula for the hydrocarbon methylpropane.
Learn more about hydrocarbons at: https://brainly.com/question/1489747
#SPJ1
If 25. 0ml of 0. 160M of NaOH are added to 50ml of 0. 100M of HCl, what is the pH of the resulting solution?
Answers
The pH of the resulting solution is 1.30 if the total volume of the solution is 25.0 ml.
The volume of solution = 25. 0ml
Molarity of NaOH = 0. 160M
Molarity of HCl = 0. 100M
The balanced equation for the reaction between NaOH and HCl is:
NaOH + HCl → NaCl + \(H_{2} O\)
The number of moles of NaOH = 0.160 M x 0.0250 L = 0.00400 mol
The number of moles of HCl = 0.100 M x 0.0500 L = 0.00500 mol
To calculate the Hydrogen concentration ions are:
[H+] = moles of H+ ions / volume of solution
[H+] = 0.00500 mol / (0.0250 L + 0.0500 L)
[H+]= 0.0500 M
To find the pH of the solution, the formula used is:
pH = \(-log_{H+}\)
pH = \(-log_{0.0500}\)
pH = 1.30
Therefore, we can conclude that the pH of the resulting solution is 1.30.
To learn more about the pH of the resulting solution
https://brainly.com/question/30911639
#SPJ4
Part 1: Name the type of chemical reaction that occurs when magnesium chloride
solution (MgCl2) reacts with sodium carbonate solution (Na2CO3).
Part 2: Explain why aluminum (AI) would react with copper chloride (CuCl2) but not with
magnesium chloride (MgCl2).
Answers
Mg is ahead of Al in the electrochemical series and more reactive than Al hence Al does not react with MgCl2.
A chemical reaction is said to have occurred when new substances are formed. The reaction between magnesium chloride solution and sodium carbonate solution is a double decomposition reaction since the cations exchange anion partners in the product;
MgCl2(aq) + Na2CO3(aq) ------> MgCO3(s) + 2NaCl(aq)
We must note that a single replacement reaction depends on the relative positions of metals in the electrochemical series. Al can react with CuCl2 because Al is more reactive than copper and ahead of Cu in the electrochemical series. On the other hand, Mg is ahead of Al in the electrochemical series and more reactive than Al hence Al does not react with MgCl2.
Lear more about electrochemical series: https://brainly.com/question/25749323
Water has the following composition: pH = 7.8 HCO32 = 85 mg/L as CaCO3 Ca²+ = 32 mg/L as CaCO3 Mg2+ = 40 mg/L as CaCO3 The following three questions pertain to this water. What is the highest theoretical concentration of Ca2+ (M) that can be dissolved at this pH in equilibrium with Ca(OH)₂(s) assuming no other calcium solids will form? Note: Don't be alarmed - it will be a large number! Ca(OH)(s) <--> Ca²+ + 2OH Kp-10:53
Answers
The first step in solving this problem is to calculate the activity product of calcium ions in the water to determine the saturation state of calcium with respect to Ca(OH)₂ (s).Then, using the solubility product (Ksp) of calcium hydroxide, we can calculate the theoretical maximum concentration of calcium ions in the water.
For Ca(OH)₂(s), the equilibrium expression is Ca(OH)(s) <--> Ca²+ + 2OH Kp-10:53The equilibrium constant, Kp-10:53, for this reaction is equal to the solubility product of Ca(OH)₂ (s) because it is an ionic solid. The Ksp of Ca(OH)₂ (s) is given as Ksp= [Ca²+][OH]². Using this, we can calculate the activity product, Q, for calcium ions in the water at equilibrium with Ca(OH)₂ (s):Q = [Ca²+][OH]²
the activity product of calcium ions in the water is:Q = [Ca²+][OH-]²= [Ca²+](1.58 x 10-8)²= 3.97 x 10-17The equilibrium constant, Kp-10:53, is equal to Ksp= [Ca²+][OH-]², so we can write:Ksp = [Ca²+](1.58 x 10-8)²Ksp/(1.58 x 10-8)² = [Ca²+]= (10-10.53)/(1.58 x 10-8)² = 3.24 x 10-6 mol/LThis is the theoretical maximum concentration of calcium ions that can exist in the water without precipitation of calcium solids. Note that this is an extremely high concentration of calcium ions.
To know more about equilibrium visit:
https://brainly.com/question/30694482
#SPJ11
If a reaction occurs, what will be the products of the unbalanced reaction
below
Cu(s) + Ni(NO3)2(aq) ➡️
A. CuNi(NO3)4(s)
B. No reaction will occur.
C. CuNi(s) + NO2(g)
D. Ni(s) + Cu(NO3)2(aq)
Answers
Answer:
B
Explanation:
Copper is lower on the activity series, so it can't replace nickel in this reaction. Therefore, no reaction will occur.
Please help fast, I will give brainliest!!

Answers
Answer:
The third one.
Explanation:
It goes from one reactant to two products.
Answer:
Reaction #3
Explanation:
Decomposition reactions always involve a singular reactant being broken down into multiple products, and the only reaction that involves the reactant being separated into multiple substances is the third reaction.
cobalt-60 is radioactive and has a half life of years. how much of a sample would be left after years? round your answer to significant digits. also, be sure your answer has a unit symbol.
Answers
To calculate the remaining amount of a cobalt-60 sample after 20 years, we can use the formula for radioactive decay:N = N₀ * (1/2)^(t / t(1/2)). Hence remaining amount of the cobalt-60 sample after 20 years is approximately 0.1891 times the initial amount
Given that the half-life of cobalt-60 is 5.27 years, we can substitute the values into the formula:
N = N₀ * (1/2)^(20 / 5.27)
Simplifying the equation:
N = N₀ * (1/2)^(3.7941)
Calculating the value inside the parentheses:
(1/2)^(3.7941) ≈ 0.1891
Now, multiplying the initial amount N₀ by the calculated value:
N = N₀ * 0.1891
Therefore, the remaining amount of the cobalt-60 radioactive isotope sample after 20 years is approximately 0.1891 times the initial amount. Please provide the unit symbol for the initial amount, and you can multiply it by the calculated value to obtain the unit symbol for the remaining amount.
Learn more about radioactive isotope here: brainly.com/question/28039996
#SPJ11
Complete question:
cobalt-60 is radioactive and has a half-life of 5.27 years. how much of a sample would be left after 20 years? round your answer to significant digits. also, be sure your answer has a unit symbol.
convert the following

Answers
643hg=64,300,000mg
2km=78740.2in
18lb=8.16466kg
If some solid sodium solid hydroxide is added to a solution that is 0.010–molar in (CH3)3CCl and 0.10–molar in NaOH, which of the following is true? (Assume the temperature and volume remain constant.)answer choicesa. Both the reaction rate and k increase.b. Both the reaction rate and k decrease.c. Both the reaction rate and k remain the same.d. The reaction rate increases but k remains the same.e. The reaction rate decreases but k remains the same.
Answers
If some solid sodium hydroxide is added to a solution that is 0.010–molar in (CH₃)₃CCl and 0.10–molar in NaOH, the reaction rate increases but k remains the same. Therefore, option D is correct.
In this scenario, when solid sodium hydroxide (NaOH) is added to a solution containing (CH₃)₃CCl and NaOH, a reaction between (CH₃)₃CCl and NaOH takes place. The balanced chemical equation for this reaction is:
(CH₃)₃CCl + NaOH ⇒ (CH₃)₃COH + NaCl
The reaction rate is determined by the concentration of the reactants. In this case, the concentration of (CH₃)₃CCl remains constant because only solid NaOH is added.
The rate constant depends on the specific reaction and the conditions under which it occurs. Since the temperature and volume remain constant, the rate constant (k) will also remain constant.
To learn more about NaOH, follow the link:
https://brainly.com/question/20573731
#SPJ12
If the ph of a solution is increased from ph 5 to ph 7, it means that the?
Answers
If the pH of a solution is increased from pH 5 to pH 7, it means that the solution has become more alkaline. The pH scale ranges from 0 to 14, with 7 being neutral, less than 7 being acidic, and greater than 7 being basic or alkaline.
A pH increase from 5 to 7 means that the concentration of hydroxide ions in the solution has increased, leading to a higher level of basicity.
A change in pH can have significant impacts on the chemical and biological systems that the solution interacts with. For example, a change in pH can alter the solubility of certain substances, alter the activity of enzymes, or affect the growth and behavior of microorganisms. It is important to carefully control the pH of solutions in various applications, such as in food and beverage processing, pharmaceutical manufacturing, and environmental management.
Find out more about pH
brainly.com/question/10954432
#SPJ4
What effect could the pollution of Groundwater have on a nearby River, Lake or Stream?
Answers
Answer:
please give me brainlist and follow
Explanation:
Contamination of ground water can result in poor drinking water quality, loss of water supply, degraded surface water systems, high cleanup costs, high costs for alternative water supplies, and/or potential health problems. The consequences of contaminated ground water or degraded surface water are often serious.
a saline solution has a concentration ratio of 0.5 milligrams of salt in 75 millilitres of solution
how many milligrams of salt will be needed to produce 180 millilitres of saline soluyhaving this same concentration
Answers
To produce 180 millilitres of a saline solution of the same concentration, we need 1.2 milligrams of salt.
It is given that a ratio of 0.5 milligrams of salt is present in 75 millilitres of solution. Let us assign a variable x to the amount of salt present in 180 millilitres of saline solution.
To maintain the same concentration we can use the method of cross-multiplication:
The following equation can be used to determine the value of x:
0.5 mg / 75 ml = x mg / 180 ml
( 0.5 × 180) / 75 = x mg
x mg = 90 / 75
Hence x is approximately equal to 1.2 mg.
To learn more about the saline solution:
https://brainly.com/question/29402636
The temperature of a 95.4 g piece of copper decreases from 48°C to 25°C when the copper releases - 849 J of heat. What is the specific heat of copper?
Answers

Is water an element
Answers
Answer:yes
Explanation:the element of the world is water,fire,air,and earth
It contains hydrogen and oxygen atoms and thus is not an element
The mass of an object is 1,000 g. It has a volume of 100 mL. What is the density of the object? D= mass/volume
Answers
Answer:
10 g/ml
Explanation:
divide mass by volume means divide 1000 by 100 and your answer will be 10
saras car is advertised to get 43 miles per gallon. Sara calculated her gas mileage over the pass month to 39 miles per gallon was Sara's percent yield og gas mileage?
Answers
Answer:
Sara's percentage yield of gas mileage is approximately 90.7%
Explanation:
Percentage yield of an process is given by the ratio of the observed, experienced (calculated), or actual yield to the theoretical yield of the process and then multiplying the result by 100%
\(Percentage \ yield = \dfrac{Actual \ yield}{Theoretical \ yield} \times 100\)
The theoretical yield of Sara's car is the mileage advertised for the car which the car has 'ideally' which is 43 miles per gallon
∴ The theoretical yield = 43 miles/gallon
The actual yield is the yield of the car Sara is able to calculate, based on how she uses the car which is 39 miles per gallon
∴ The actual yield = 39 miles/gallon
Sara's percentage yield of gas mileage for the car, % yield, therefore is given as follows;
% yield = (39 miles/gallon)/(43 miles/gallon) × 100 ≈ 90.7%
Sara's percentage yield of gas mileage, % yield ≈ 90.7%.
PLEASE HELP ASAP
Predict the missing component
in the nuclear equation.
135 Cs
135Ba + X
55
56
A
00
B
He
Lee
07
Answers
The missing component "X" in the nuclear equation is hydrogen (H).
Based on the given information, the nuclear equation is:
135Cs → 135Ba + X
The missing component represented by "X" can be determined by examining the atomic numbers and mass numbers involved in the equation.
The atomic number of 135Cs is 55, and the atomic number of 135Ba is 56. Therefore, the sum of atomic numbers on both sides of the equation should be equal to maintain charge balance.
Since 55 + 1 = 56, the missing component "X" must have an atomic number of 1. An element with atomic number 1 is hydrogen, which is represented by the symbol "H."
Therefore, the missing component "X" in the nuclear equation is hydrogen (H).
Learn more about nuclear reaction, here:
https://brainly.com/question/13315150
#SPJ12
4. Write the complete electron-configuration
notation, the noble-gas notation, and the orbital
notation for the following elements:
a. carbon b. neon c. sulfur
Answers
a. Carbon:
Complete electron configuration notation: 1s^2 2s^2 2p^2
Noble gas notation: [He] 2s^2 2p^2
Orbital notation: ↑↓ 1s ↑↓ 2s ↑↓ 2p
b. Neon:
Complete electron configuration notation: 1s^2 2s^2 2p^6
Noble gas notation: [He] 2s^2 2p^6
Orbital notation: ↑↓ 1s ↑↓ 2s ↑↓ 2p
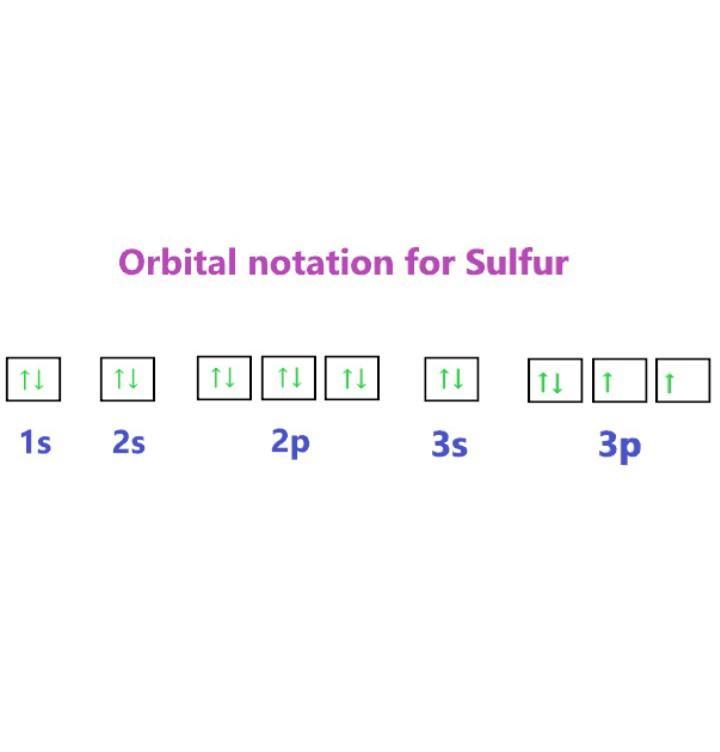
c. Sulfur:
Complete electron configuration notation: 1s^2 2s^2 2p^6 3s^2 3p^4
Noble gas notation: [Ne] 3s^2 3p^4
Orbital notation: ↑↓ 1s ↑↓ 2s ↑↓ 2p ↑↓ 3s ↑↓ 3p
a. Carbon :
Atomic number = 6
Electronic configuration notation : 1s² 2s² 2p²
Noble-gas notation : [He] 2s² 2p²
Orbital notation : Refer to the attachment.
b. Neon
Atomic number : 10
Electronic configuration notation : 1s² 2s² 2p⁶
Noble-gas notation : [He] 2s² 2p⁶
Orbital notation : Refer to the attachment.
c. Sulfur
Atomic number : 16
Electronic configuration notation : 1s² 2s² 2p⁶ 3s² 3p⁴
Noble-gas notation : [Ne] 3s² 3p⁴
Orbital notation : Refer to the attachment.


Explain why the mass of the powder is greater than the mass of the magnesium metal.
Answers
Answer:
because when it burns reacts with oxygen forming mg oxide
The statement ""the soup is very salty "" is a/an
Answers
Does trans-oleic acid have a higher or lower melting point than cis-oleic acid? Explain Which triacylglycerol yields more energy on oxidation: one containing three resides of linolenic acid or three residues of stearic acid?
Answers
Trans-oleic acid has a higher melting point than cis-oleic acid and a triacylglycerol containing three residues of stearic acid yields more energy upon oxidation compared to one containing three residues of linolenic acid.
Trans-oleic acid has a higher melting point than cis-oleic acid. This is because the trans configuration results in a more linear structure, allowing the molecules to pack more closely together, leading to stronger intermolecular forces and a higher melting point.
A triacylglycerol containing three residues of stearic acid yields more energy upon oxidation compared to one containing three residues of linolenic acid.This is because stearic acid is a saturated fatty acid, which means it has a higher carbon-to-hydrogen ratio, leading to more energy release upon oxidation. Linolenic acid, on the other hand, is an unsaturated fatty acid and has a lower carbon-to-hydrogen ratio, resulting in less energy release upon oxidation.
For more questions on Trans-oleic acid:
https://brainly.com/question/14974487
#SPJ11
Trans-oleic acid has a higher melting point than cis-oleic acid. Three residues of stearic acid yield more energy on oxidation than three residues of linolenic acid due to the higher number of carbon atoms and absence of double bonds.
Trans-oleic acid has a higher melting point than cis-oleic acid due to its straighter shape, which allows for closer packing and stronger intermolecular forces. In contrast, cis-oleic acid has a kink in its structure due to the cis double bond, which results in weaker intermolecular forces and a lower melting point.
Stearic acid has a higher number of carbon atoms (18) and lacks double bonds, making it a saturated fatty acid. Saturated fatty acids can pack more closely together, resulting in stronger intermolecular forces and a higher energy yield upon oxidation. In contrast, linolenic acid is an unsaturated fatty acid with three double bonds, making it a polyunsaturated fatty acid. The presence of double bonds causes kinks in the fatty acid chains, making it more difficult for them to pack together, resulting in weaker intermolecular forces and a lower energy yield upon oxidation.
Learn more about Trans-oleic acid here :
brainly.com/question/14974487
#SPJ11
assuming that the cell membrane is not permeable to its ions, is a 7.26×10-2 m aqueous solution of chromium(ii) iodide, cri2, hypertonic, hypotonic or isotonic to red blood cells?
Answers
A 7.26 × \(10^{-2}\) M aqueous solution of chromium(II) iodide, \(CrI_{2}\), is hypertonic to red blood cells.
1. Red blood cells are typically isotonic with solutions that have an osmolarity of approximately 0.3 Osm/L (300 mOsm/L), which corresponds to a 0.15 M NaCl solution.
2. To determine the osmolarity of the \(CrI_{2}\) solution, we must first identify the number of particles (ions) that will be released into the solution when it dissolves. In this case, one \(CrI_{2}\) molecule dissociates into one \(Cr^{2+}\) ion and two I- ions.
3. Next, multiply the molarity of the \(CrI_{2}\) solution (7.26×\(10^{-2}\) M) by the number of ions it dissociates into (1 + 2 = 3 ions). This gives us an osmolarity of 7.26×\(10^{-2}\) M × 3 = 2.178×\(10^{-1}\) Osm/L (217.8 mOsm/L).
4. Compare the osmolarity of the \(CrI_{2}\) solution to that of red blood cells (217.8 mOsm/L vs. 300 mOsm/L). Since the \(CrI_{2}\) solution has a higher osmolarity, it is hypertonic to red blood cells.
To know more about Tonicity of solutions:
https://brainly.com/question/14266650
#SPJ11
In naming a binary molecular compound,the number of atoms of each element present in the molecular is indicated by?
Answers
Using prefixes before the name of each element, the number of atoms of each element present in the molecular is indicated.
A prefix is placed before the name of the element when naming a binary compound to indicate how many atoms are present.For example:In carbon dioxide which is CO2 (prefix di- meaning 2)In carbon tetrachloride which is CCl4 (prefix tetra- meaning 4) In N2O5, the name is dinitrogen pentoxide (prefix di- and pent- meaning 2 and 5)It depends on the number of atoms present in a compound.A binary compound is a compound composed of two elements.To learn more about binary compounds visit:
brainly.com/question/7960132
#SPJ1
Can someone help me thank you. I have to look at each picture and determine if the circuits shown are series circuits or parallel circuits. Explain how you know.

Answers
Answer:
I believe it may be-
1. Series
2. Parallel
3. Parallel
4. Series
Explanation:
In a series circuit, electricity only has one path to follow while a parallel circuit has more than one path to follow.
solve the quadratic equation 2x^2+13x=15 by method of completing the square
Answers
Answer:
x = 1, -7.5
Explanation:
2x² + 13x = 15
Divide the left side of the equation by 2
2(x² + 6.5x) = 15
Divide 6.5 by 2 and square the quotient
6.5/2 = 3.25
3.25² = 10.5625
Add 10.5625 to the left side
2(x² + 6.5x + 10.5625) = 15
Since you have a 2 outside the parentheses, you will be adding 10.5625•2 to the right side.
10.5625 • 2 = 21.125
2(x² + 6.5x + 10.5625) = 36.125
To factor (x² + 6.5x + 10.5625), add b/2 to x
b/2 = 6.5/2 = 3.25
2(x + 3.25)² = 36.125
Divide by 2
(x + 3.25)² = 18.0625
Square root.
(x + 3.25) = √18.0625
x + 3.25 = ±4.25
Subtract 3.25.
x = 4.25 - 3.25 = 1
x = -4.25 - 3.25 = -7.5
x = 1, -7.5
What mass of mgF2 is needed to produce 1. 4 mol of liF
Answers
To produce 1.4 mol of LiF, we need 0.7 mol of MgF2. This corresponds to a mass of 43.6 grams of MgF2, based on the molar mass of MgF2.
Summary of Calculations for Producing LiF with MgF2.We're given that we need to produce 1.4 mol of LiF, and we're asked to find out how much MgF2 is needed for this reaction. We can start by writing out the balanced chemical equation for the reaction:
MgF2 + 2 LiF → 2 LiF + MgF2
From this equation, we can see that 1 mol of MgF2 reacts with 2 mol of LiF to produce 1 mol of MgF2 and 2 mol of LiF. This means that the stoichiometry of the reaction is 1:2 for MgF2 and LiF. In other words, for every 1 mol of MgF2 that reacts, 2 mol of LiF is produced.
So, if we want to produce 1.4 mol of LiF, we need to use stoichiometry to figure out how much MgF2 we need. We can use a conversion factor that relates the number of moles of LiF to the number of moles of MgF2:
1.4 mol LiF × (1 mol MgF2 / 2 mol LiF) = 0.7 mol MgF2
This tells us that we need 0.7 mol of MgF2 to produce 1.4 mol of LiF.
To convert from moles to grams, we can use the molar mass of MgF2, which is 62.3 g/mol. Multiplying the number of moles by the molar mass gives us the mass of MgF2 needed:
0.7 mol MgF2 × 62.3 g/mol MgF2 = 43.6 g MgF2
Therefore, we need 43.6 grams of MgF2 to produce 1.4 mol of LiF.
To know more about molar mass, visit:https://brainly.com/question/12127540
#SPJ4