Suppose the galvanic cell sketched below is powered by the following reaction: Mg(s)+PdSO4(aq) MgSO4(aq)+Pd(s) e- E1 E2 sí S2 Write a balanced equation for the half-reaction that happens at the cathode of this cell. Write a balanced equation for the half-reaction that happens at the anode of this cell. Of what substance is El made?
Answers
The complete balanced half-cell reaction is as follows:
Mg(s)+PdSO4(aq)---->MgSO4(aq)+Pd(s)
S1 solution= MgSO4 (magnesium sulfate) and S2 solution= PdSO4 (Palladium (II) sulfate)
A chemical reaction that takes place between an oxidizing substance and a reducing substance. The oxidizing substance loses electrons in the reaction, and the reducing substance gains electrons. Oxidation numbers, which are assigned to atoms in molecules by assuming that all bonds to the atoms are ionic. An increase in oxidation number during a reaction corresponds to oxidation, while a decreases corresponds to reduction.
Cathode: Pd^2+(aq)+2e- ---> Pd(s)
At cathode, reduction (gain of e- ) occurs.
At anode: Mg(s) ----> Mg^2+(aq)+2e-
Anode, oxidation (loss of e-) occurs
E1= Mg; (as left side of cell is anode)
E2= Pd; (as right side of cell is cathode)
S1 solution= MgSO4 (magnesium sulfate)
S2 solution= PdSO4 (Palladium (II) sulfate)
To learn more oxidation number check the link below:
https://brainly.com/question/27239694
#SPJ4
Related Questions
Describe how to prepare 400 grams of a 15% (mass/mass) aqueous solution of KBr.
Answers
Dissolve 60g of potassium bromide in 340g of water to produce 15% (mass/mass) aqueous solution of potassium bromide.
Here we have to prepare a total of 400 g of solution. Aqueous solution means the solvent we use here is water.
So to prepare 400 g of 15% aqueous solution of potassium bromide, we need to find out how many grams of potassium bromide need to be dissolved in water and how many grams of water must be used.
Here the weight percent is given, that is 15%
15/100 = weight of potassium bromide/ 400 g
0 .15 = weight of potassium bromide / 400
weight of potassium bromide needed = 0.15 × 400
= 60 g
So, we calculated the required amount of potassium bromide as 60 grams. The total weight of the solution to be made is 400 grams.
So amount of water required = 400 - 60
= 340 g
So we need to mix 60 grams of potassium bromide in 340 grams of water to get a 15% (mass/mass) aqueous solution.
For further information about preparing aqueous solutions, please refer
https://brainly.com/question/13684060
Element Q has three isotopes. It is 18.75% -318, 78.26% Q-319, and 2.99% Q-320; The masses of the isotopes are 317.98 amu, 318.98 amu, and 320.026 amu respectively. What is the average atomic mass of the element Q
Answers
Answer:
Explanation:
ye
a chemist wishes to analyze the caco3 content of an antacid tablet by reaction with hcl. the tablet is weighed, and the volume of hcl required to react with the tablet is measured. which of the following statements correctly describe the assumptions that must be made in order to draw a scientifically valid conclusion from the data? (select all that apply.)
Answers
It is anticipated that none of the other compounds in the antacid tablet would interact with the HCl. It is required to assume that it is feasible to fairly accurately estimate the volume of HCl. The chemist must evaluate more than one tablet to obtain a more accurate result. Hence all the statements are true.
In order to treat indigestion and heartburn, antacids work to balance out the acid in your stomach. Without a prescription, you can get them from pharmacies and stores as liquid or chewable tablets.
Calcium carbonate is used to treat illnesses brought on by excessive stomach acid, such as heartburn, indigestion, upset stomach, and others. Lowering the level of gastric acid. It is a member of the antacid class of drugs. Additionally, it might be applied to raise your body's calcium levels. Calcium is a mineral that is crucial for developing strong bones and keeping the heart healthy.
To know more about antacids
brainly.com/question/28187760
#SPJ4
A chemist wants to use a reaction with Hcl to determine the caco3 concentration of an antacid tablet. The volume of Hcl needed to react with the pill is calculated after weighing the tablet. Which of the following statements most accurately describes the presumptions that need to be made in order to interpret the data in a way that is scientifically sound? (Check every box that applies.)
1. It is expected that the HCl won't interact with any other ingredients in the antacid tablet.
2. It is necessary to presume that it is possible to quantify the volume of HCl fairly accurately.
3. To get a more precise result, the chemist needs to examine more than one tablet.
Objects that have a higher density than water can sometimes be observed floating on water. which property of water explains this phenomenon?
Answers
Answer: Under what condition an object having density greater than water will float on water?
If an object is more dense than water it will sink when placed in water, and if it is less dense than water it will float. Density is a characteristic property of a substance and doesn't depend on the amount of substance.
4. Solve the following heat flow problem, being sure to show all your work (you may either type your
work or insert an image): Find the specific heat of 402 grams of graphite that absorbs 1136) of heat
energy as it changes temperature from 26°C to 30°C.
Answer should be 0.7J/gC
Answers
Answer:
0.70 J/g.°C
Explanation:
Step 1: Given data
Mass of graphite (m): 402 gHeat absorbed (Q): 1136 JInitial temperature: 26°CFinal temperature: 30 °CSpecific heat of graphite (c): ?Step 2: Calculate the specific heat of graphite
We will use the following expression.
Q = c × m × ΔT
c = Q / m × ΔT
c = 1136 J / 402 g × (30°C - 26°C)
c = 0.70 J/g.°C
{ PLEASE HELP} Lapis is studying the relationship between the energy transferred by electromagnetic waves and their wavelengths using this diagram.
How does she describe this relationship? Select all that apply.
Lapis is studying the relationship between the energy transferred by electromagnetic waves and their wavelengths using this diagram.
How does she describe this relationship? Select all that apply.
As the wavelength increases, the energy increases.
Radio waves have a small wavelength and a large amount of energy.
As the wavelength increases, the energy decreases.
As the wavelength decreases, the energy decreases.
Gamma rays have a large wavelength and a small amount of energy.
Gamma rays have a small wavelength and a large amount of energy.
Answers
This link between the energy carried by electromagnetic waves and their wavelengths is described as increasing as the wavelength decreases.
What do electromagnetic waves transmit?In electromagnetic waves, the electric energy is equal to the magnetic energy. The energy delivered by electromagnetic waves per unit area is represented by the Poynting vector.
Which of the following electromagnetic wave propagation techniques can be used?Electromagnetic waves can travel in three different ways: via the Earth's atmosphere, into space, and through the sky. Radio waves are electromagnetic waves with frequencies between a few Hz to approximately 1011 Hz. Magnetic resonance imaging (MRI), radiofrequency ablation (RFA), utilised in cardiology, and tumour therapy are the three primary medical uses for EMFs.
To know more about electromagnetic waves visit:-
https://brainly.com/question/2995167
#SPJ1
Which actions can be taken to plan for a drought check all that apply
Answers
Answer:
Can you attach a picture?
Explanation:
Help!!! A radio station transmits its signal at 93.1 MHz. What is the wavelength signal ?

Answers
Answer:
3.22m
Explanation:
c=3×10^8m/s=speed of the light
lambda= wavelength=?
frequency=?
lambda=c÷frequency
lambda=(3×10^8)÷(93.1×10^6)
lambda=3.22m
how would to obtain a representative sample of vitamin c in a bottle of vitamin c tablets?
Answers
To obtain a representative sample of vitamin C in a bottle of vitamin C tablets, follow these steps:
1. Thoroughly mix the tablets: Before taking any samples, make sure the tablets in the bottle are well-mixed. You can do this by shaking the bottle or turning it upside down a few times. This will help to ensure that any potential variations in the tablets are distributed evenly.
2. Determine the sample size: To obtain a representative sample, you need to decide how many tablets to test. The larger the sample size, the more accurate the results will be. You can use a sample size calculator or consult a statistician to help you determine the appropriate sample size based on the desired confidence level and margin of error.
3. Randomly select the tablets: To ensure that your sample is truly representative, you must randomly select the tablets. You can do this by assigning a number to each tablet and using a random number generator to choose the ones you'll test. Alternatively, you can blindly pick tablets without looking at them.
4. Crush and homogenize the tablets: Combine the selected tablets in a clean mortar and pestle or use a tablet crusher. Crush the tablets into a fine powder, and mix it thoroughly to create a homogeneous mixture. This will ensure that your sample is representative of the entire bottle.
5. Extract vitamin C: Follow a standard vitamin C extraction protocol using an appropriate solvent, such as a solution of metaphosphoric acid or oxalic acid. Filter the solution to remove any particulate matter.
6. Quantify the vitamin C content: To determine the concentration of vitamin C in your sample, you can use methods like titration (with iodine or 2,6-dichloroindophenol), High-Performance Liquid Chromatography (HPLC), or UV-visible spectrophotometry.
7. Calculate the average and confidence interval: Analyze the data you've collected to determine the average vitamin C content per tablet in your sample. Calculate the confidence interval to provide an estimate of the range within which the true value of the average vitamin C content is likely to fall.
By following these steps, you can obtain a representative sample of vitamin C in a bottle of vitamin C tablets and gain an understanding of the overall quality and consistency of the product.
If 28 grams of N reacts completely with 12 grams of H2, then how many grams of NH6 will you end up with? ___________ grams
Answers
Answer:
40 Grams
Explanation:
add the 28 and 12 NH6 and H2 doesn't matter for the grams because they are way way less then grams in weight
Answer:40
fjfbydgbsyfbgybg
Fluoride is often added to water as sodium fluoride (NaF). What is the mass percent composition of in NaF? How many grams of NaF must be added to 1500 L of water to fluoridate it at a level of 0.7 mg
Answers
The Mass percent composition of F - in NaF is 45.25% .
2.323 g must be added to 1500 L of water to fluoridate it at a level of 0.7 mg.
What is mass percent composition?Mass percent composition describes the relative quantities of elements in a chemical compound.
The given data is:
Volume of water = 1500 L
Amount of F- ion = 0.7 mg/L
Atomic weight of F = 19 g/mol
Number of moles of F- in 1 L of water = 0.7 x 10⁻³ g/19 g mol⁻¹ = 3.684x 10⁻⁵ moles
Therefore, the number of moles of F- in 1500 L of water = 3.684 x 10⁻⁵ x 1500 = 0.0553 moles
1 mole of NaF has 1 mole of Na+ and 1 mole of F-
hence, the number moles of NaF required = 0.0553 moles
Molar mass of NaF = 23 +19 = 42 g/mol
Mass of NaF required = 0.0553 moles x 42 g/mol = 2.323 g
Learn more about Mass percent composition at: https://brainly.com/question/26150306
#SPJ1
2. a) what is the empirical formula of an ingredient in bufferin table that has the percent composition C1:4.25%, 0:56 93% and Mg: 28.83 % by mass
b) An analysis of sample of an organic compound shows that it contains 39.9 % C, 6.9% H, and 53,2% 1. calculate the empirical formula of the compound
2. the relative molecular mass is 60 what is the molecular formula of the compound?
Answers
Explanation:
a)Take percentages and divide by mole wt ( from periodic table) of the corresponding element
C 14.25 / 12 = 1.1875
O 56.93 / 15.99 = 3.56
Mg 28.83/24.3 = 1.186 Divide by the smallest number
C 1.1875/1.186 = 1
O 3.56 / 1.186 = 3
Mg 1.186 / 1.186 = 1
C O3 Mg commonly written as Mg CO3 ( magnesium carbonate)
The question is listed in the image attached.

Answers
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
What will happen to the following equilibrium if Cl2
was removed ?
4HCI(9) + O2(9) = 2012(9) + 2H20(9)
Answers
The following equilibrium will shift in the direction of the product:
Further explanationGiven
Reaction
4HCl + O₂ → 2H₂O + 2Cl₂
Cl₂ was removed
Required
Equilibrium changes
Solution
Reaction = - action
adding the products ⇒ Shifts in the direction of the reactants
reducing the products ⇒Shifts in the direction of the products
Cl₂ as a product, so if Cl₂ is taken or reduced, the reaction will try to maintain system equilibrium by moving to the right (product formation) ⇒ Shift in the direction of the product:
What is 188.5°F in Celsius?
Answers
Answer:
That would be 86.944 Celsius
Explanation:
(188.5°F − 32) × 5/9 = 86.944°C
Answer: \(86.944444\)°C
Explanation: (188.5°F − 32) × 5/9 = 86.944°C
PLEASE HELP ! :D
WILL GIVE BRAINLIEST

Answers
Number 3
The question is: What are the formulas for Ions and Compunds formed?
Ca, CL
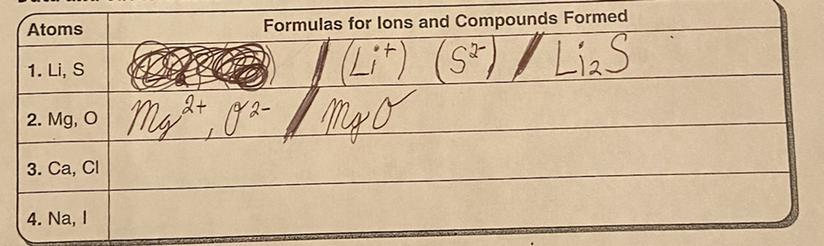
Answers
Answer:
hope this help u! sorry late answer

3.544g of hydrogen gas contains
Answers
Answer:
1.772 mols
Explanation:
Because hydrogen exists as a diatomic molecule in gaseous state, that's (H2), so then it's molecular mass is 1 ×2= 2g/mol.
we can now calculate for the number of moles, so
n=m/Mr
n=3.544/2
n=1.772mols
If a gas sample has a pressure of 74 ka at 87 L, what would the new volume be if the pressure changed to 929 kPa?
Answers
Answer:
La ley de los gases ideales relaciona cuatro propiedades macroscópicas de los gases ideales (presión, volumen, número de moles y temperatura). Si conocemos los valores de tres de estas propiedades, podemos utilizar la ley de los gases ideales para conocer la cuarta. En este video, usaremos la ley de los gases ideales para resolver el número de moles (y en última instancia de moléculas) en una muestra de un gas
Explanation:
Power plants that discharge warm water into rivers have a negative effect on aquatic
life. This is because the higher water temperature ?
Answers
Answer: hope this helps
Explanation: Reduces the amount of dissolved oxygen in the water, making it challenging for aquatic life to breathe. Fish require more oxygen because their metabolic rate increases in warm water. Additionally, it may alter the timing of aquatic organisms' life cycles and breeding patterns, as well as the rates at which some species grow. The food chain may be disrupted, and the health of the ecosystem as a whole may suffer. In order to reduce their negative effects on aquatic life, power plants that release warm water into rivers need to be carefully regulated.
PLEASE HELP!!1.A student collected the following data for a fixed volume of gas: Temperature (⁰C)Pressure (mm of Hg) 10726 20750 40800 70880 100960 150??? Fill in the missing data point. Show all calculations leading to an answer.2.You are given a clear solution of KNO3. Using 3 – 4 sentences (in your own words) explain how you would determine if the solution is unsaturated, saturated or supersaturated.3.Explain why gasoline will not dissolve in water.
Answers
Explanation:
First, let's remember the concepts of an unsaturated, saturated, and supersaturated solution:
- An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved.
- A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving.
- A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
Answer:
In this case, for KNO3, we can say:
- If we add KNO3 (crystal) to a solution (for example, water), and it dissolves completely, it would be an unsaturated solution.
- If the crystal doesn't grow, the solution is saturated.
- If we see a lot of crystals in the solution, we're going to have a supersaturated solution.
What mass (grams) of sodium sulfate would be formed by the complete reaction of 120.0 grams of sodium hydroxide?
Answers
Answer:
The mass of sodium sulfate formed by the comolete reaction of 120.0 grams of sodium hydroxide is 142.04 grams.
Explanation:
The balanced chemical equation for the reaction between sodium hydroxide and sulfuric acid is:
2NaOH + H2SO4 -> Na2SO4 + 2H2O
From the equation, we can see that 2 moles of sodium hydroxide react with 1 mole of sulfuric acid to form 1 mole of sodium sulfate. We can use this information, along with the molar masses of the compounds, to calculate the mass of sodium sulfate formed.
First, we need to convert the given mqss of sodium hydroxide to moles. The molar mass of sodium hydroxide is 40.00 g/mol, so:
Moles of NaOH = Mass of NaOH / Molar mass of NaOH
Moles of NaOH = 120.0 g / 40.00 g/mol
Moles of NaOH = 3.00 mol
Next, we can use the mole ratio from the balanced equation to calculate the moles of sodium sulfate formed:
Moles of Na2SO4 = Moles of NaOH / 2
Moles of Na2SO4 = 3.00 mol / 2
Moles of Na2SO4 = 1.50 mol
Finally, we can convert the moles of sodium sulfate to grams using its molqr mass of 142.04 g/mol:
Mass of Na2SO4 = Moles of Na2SO4 x Molar mass of Na2SO4
Mass of Na2SO4 = 1.50 mol x 142.04 g/mol
Mass of Na2SO4 = 213.06 g
Therefore, the mass of sodium sulfate formed by the complete reaction of 120.0 grams of sodium hydroxide is 213.06 grams.
A sample of ore containing the mineral tellurite, TeO2 , was dissolved in acid. The resulting solution was then
reacted with a solution of K2Cr2O7 to form telluric acid, H2TeO4 . The unbalanced chemical equation for the
reaction is given below.
Answers
The molecule or ion that is being oxidized in the reaction is TeO2. Ceramics may occasionally be colored using tellurium dioxide.
TeO2 does water dissolve TeO2?TeO2 is soluble in strong acids, alkali metal hydroxides, and only very little water. Due to its amphoteric nature, it can operate as either an acid or a base depending on the solution it is in. It interacts with bases to form tellurites and acids to create tellurium salts.
TeO2's form is what?Due to the presence of 6 e-pairs surrounding the center atom, 6 atoms bound to it, and 0 lone pairs, this molecule is octahedral.
To know more about telluric acid(TeO2) visit :-
brainly.com/question/29397392
#SPJ4
The complete question is:
A sample of ore containing the mineral tellurite, TeO2 , was dissolved in acid. The resulting solution was then
reacted with a solution of K2Cr2O7 to form telluric acid, H2TeO4 . The unbalanced chemical equation for the
reaction is given below.
. . . TeO2(s) + . . . Cr2O72−(aq) + . . . H+(aq) → . . . H2TeO4(aq) + . . . Cr3+(aq) + . . . H2O(l)
(a) Identify the molecule or ion that is being oxidized in the reaction.
Wine goes bad soon after opening because the ethanol (CH3CH2OH) dissolved in it reacts with oxygen (O2) gas to form water and aqueous acetic acid (CH3COOH), the main ingredient in vinegar. Calculate the moles of oxygen needed to produce 0.080 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
Answers
The number of mole of oxygen needed is of 0.080 mole.
To solve this question, we'll begin by writing the balanced equation for the reaction. This is illustrated below:
CH₃COOH + 2O₂ —> CO₂ + 2H₂OFrom the balanced equation above,
2 moles of O₂ reacted to produce 2 moles of H₂O.
Finally, we shall determine the number of mole of O₂ needed to produce 0.080 mole of H₂O. This can be obtained as follow:
From the balanced equation above,
2 moles of O₂ reacted to produce 2 moles of H₂O.
Therefore,
0.080 mole of O₂ will also react to produce 0.080 mole of H₂O.
Thus, 0.080 mole of oxygen, O₂, is needed for the reaction.
Learn more: https://brainly.com/question/1563415
identify the answer choice that lists 62co, 64ni, 61cu, and 64zn in order of increasing number of neutrons
Answers
62Co, 61Cu, 64Ni, 64Zn . the answer choice that lists 62co, 64ni, 61cu, and 64zn in order of increasing number of neutrons The number of neutrons equals.
neutrons the difference between the atomic mass number (M) and the atomic number (Z). Copper has an atomic mass of 63.5 and an atomic number of 29. - The atomic number of a neutral element is always equal to the amount of electrons or protons present. As a result, the copper atom has 34 neutrons.Cu-61 < Zn-64 < Co-62 < Ni-64. the answer choice that lists 62co, 64ni, 61cu, and 64zn in order of increasing number of neutrons The number of neutrons equals. Copper exists in nature primarily as two isotopes: 6329Cu and 6529Cu. An atom's atomic number is equal to the number of protons in its nucleus or the number of electrons in an electrically neutral atom. A sodium atom, for example, contains 11 electrons and 11 protons. As a result, the atomic number of the Na atom Equals number.
learn more about neutrons here:
https://brainly.com/question/28992636
#SPJ4
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
What is the change in enthalpy when 90.6 g
of steam at 100◦C is converted to liquid water
at the same temperature and pressure? The
heat of vaporization of water is 40.7 kJ/mole.
Answers
The change in enthalpy when 90.6 g of steam at 100◦C is converted to liquid water at the same temperature and pressure is 204.7 KJ
How do i determine the change in enthalpy?First, we shall obtain the number of mole water converted to steam. details below:
Mass of water = 90.6 grams Molar mass of water = 18 g/mol Mole of water =?Mole = mass / molar mass
Mole of water = 90.6 / 18
Mole of water = 5.03 moles
Finally, we shall determine the change in enthalpy. Details below:
Mole of water (n) = 5.03 molesHeat of vaporization (ΔHv) = 40.7 KJ/molChange in enthalpy (ΔH) =?ΔH = n × ΔHv
ΔH = 5.03 × 40.7
ΔH = 204.7 KJ
Thus, we can conclude that the change in enthalpy is 204.7 KJ
Learn more about enthalpy change:
https://brainly.com/question/24170335
#SPJ1
what are the three important properties of acids
Answers
7) How many molecules of CO2 are in 2.5 L at STP?
Answers
By using the ideal gas law and Avogadro's number, we find that there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
To determine the number of molecules of CO2 in 2.5 L at STP (Standard Temperature and Pressure), we can use the ideal gas law and Avogadro's number.
Avogadro's number (N_A) is a fundamental constant representing the number of particles (atoms, molecules, ions) in one mole of substance. Its value is approximately 6.022 × 10^23 particles/mol.
STP conditions are defined as a temperature of 273.15 K (0 °C) and a pressure of 1 atmosphere (1 atm).
First, we need to convert the volume from liters to moles of CO2. To do this, we use the ideal gas law equation:
PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we have STP conditions, we can substitute the values:
(1 atm) × (2.5 L) = n × (0.0821 L·atm/(mol·K)) × (273.15 K).
Simplifying the equation:
2.5 = n × 22.4149.
Solving for n (the number of moles):
n = 2.5 / 22.4149 ≈ 0.1116 moles.
Next, we can calculate the number of molecules using Avogadro's number:
Number of molecules = n × N_A.
Number of molecules = 0.1116 moles × (6.022 × 10^23 particles/mol).
Number of molecules ≈ 6.72 × 10^22 molecules.
Therefore, there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
For more such questions on ideal gas law visit:
https://brainly.com/question/27870704
#SPJ8