Answers
Answer:
C. Aqueous Solutions
General Formulas and Concepts:
Aqueous Solutions'
DissociationSolvents and SolutionsExplanation:
Water is the universal solution that solvents are dissolved in. Whenever anything is dissolved in water, it will be an aqueous solution.
Strong ionic solutions dissolve into their ions when dissolved in water.
Weak acids and bases don't fully dissociate in water.
Related Questions
A chemical reaction occurs when we see a chemical?
Answers
Answer:
A chemical reaction is usually accompanied by easily observed physical effects, such as the emission of heat and light, the formation of a precipitate, the evolution of gas, or a color change.
PLEASE ANSWER QUICKLY!!
100 NaNO3
90
Solute per 100 g of H₂O (g)
0,80
NH,CI
70 KNO3
60
50
40
30
20
10
0
0 10 20 30 40 50 60 70 80 90 100
Temperature (°C)
KCIO3
60 g KNO3 has
been added to
100 g H₂O at
30 °C. What
type of solution
is this?
A. unsaturated
B. saturated
C. supersaturated

Answers
If 60 grams of the substance are added to 100 g of water, the solution can be categorized as supersaturated.
How saturated is this solution?The graph shows the number of grams that can be dissolved in 100 grams of water at different temperatures. In general, solubility increases with temperature.
According to the graph, at a temperature of 30°C, it is possible to dissolve a total of 48 to 49 grams of \(KNO_{3}\). This information implies that if we add 60 grams at this temperature not all the substance would be dissolved, and therefore the solution would be supersaturated.
Learn more about solubility in https://brainly.com/question/31493083
#SPJ1
Which most likely indicates a chemical change has occurred?
a solid substance becoming larger
a solid melting and becoming a liquid
a green liquid becoming a red liquid
a liquid freezing and becoming a solid
Answers
Answer:
A green liquid becoming a red liquid.
Explanation:
If the color change is unexpected it is a chemical change.
(If 2 clear liquids make a black, red, green, ect color it is a chemical change)
no normal color mix makes green turn red.
Nonmetallic elements form ions by _______ valence electrons to complete their outer shell. The ______ valence electrons an element has in its outer shell, the easier it is to complete. The _______ electron shells an element has, the easier it is to fill its outermost shell. Reactivity in nonmetals _______ as you go from left to right in a group, and ______ as you go from top to bottom.
Answers
Answer:
Nonmetallic elements form ions by gaining valence electrons to complete their outer shell.
The more valence electrons an element has in its outer shell, the easier it is to complete.
The fewer electron shells an element has, the easier it is to fill its outermost shell.
Reactivity in nonmetals increases as you go from left to right in a group, and
decreases as you go from top to bottom.
Explanation:
Nonmetallic elements form ions by gaining valence electrons to complete
their outer shell.
The more valence electrons an element has in its outer shell, the easier it is to complete.
The fewer electron shells an element has, the easier it is to fill its outermost shell.
Reactivity in nonmetals increases as you go from left to right in a group, and decreases as you go from top to bottom.
When there are more valence electrons then it makes it easy for the element
to complete its outermost shell as against if it was less as it would be harder
due to a bigger number of electrons needed.
Non metals are elements which accepts valence electrons to complete its
outermost shell and becomes negatively charged when this happens.
When an element has fewer electron shells then filling the outermost shell
will be easier as the electron shells require a lesser number of electrons and
increases as the number of shells increases.
This is because the tendency to accept electrons increases from left to right
and decreases down the group.
Read more on https://brainly.com/question/17266205
In the reaction 2 CO(g) + O2 (g) ® 2 CO2 (g), what is the ratio of moles of oxygen used to moles of CO2 produced?
Answers
Answer:
2 answers
Explanation:
Why the ratio of dioxygen to carbon dioxide is 1:2 on the basis of moles, i.e. on the basis of number of particles .
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced.
reaction
4 HF (9) + SiO₂ (s) → SiF4(9) + 2 H₂O(g)
2 Cl(aq) + 2 H₂O 2OH(aq) + H₂(g) + Cl₂(9)
-
H₂S(aq) + 2NaOH(aq) → Na₂S(aq) + 2 H₂O(
2 H₂(g) + O₂(g) 2 H₂O(g)
-
oxidized
O
O
O
highlighted atom is being...
O
reduced
O
O
O
neither oxidized
nor reduced
O
O
O
S
Answers
- In reactions 1 and 2, the highlighted atom (oxygen) is reduced.
- In reaction 3, the highlighted atom (sulfur) is neither oxidized nor reduced.
- In reaction 4, the highlighted atom (oxygen) is reduced.
In the given chemical reactions, we need to identify whether the highlighted atom is being oxidized or reduced. Let's analyze each reaction individually:
Reaction 1: 4 HF (g) + SiO₂ (s) → SiF₄ (g) + 2 H₂O (g)
In this reaction, the highlighted atom is oxygen (O). Oxygen in SiO₂ undergoes a change in oxidation state from -2 to 0 in SiF₄. Therefore, the highlighted atom (oxygen) is reduced.
Reaction 2: 2 Cl (aq) + 2 H₂O (l) → 2 OH (aq) + H₂ (g) + Cl₂ (g)
In this reaction, the highlighted atom is oxygen (O). Oxygen in H₂O undergoes a change in oxidation state from -2 to -1 in OH. Therefore, the highlighted atom (oxygen) is reduced.
Reaction 3: H₂S (aq) + 2 NaOH (aq) → Na₂S (aq) + 2 H₂O (l)
In this reaction, the highlighted atom is sulfur (S). Sulfur in H₂S undergoes a change in oxidation state from -2 to -2 in Na₂S. Therefore, the highlighted atom (sulfur) is neither oxidized nor reduced.
Reaction 4: 2 H₂ (g) + O₂ (g) → 2 H₂O (g)
In this reaction, the highlighted atom is oxygen (O). Oxygen in O₂ undergoes a change in oxidation state from 0 to -2 in H₂O. Therefore, the highlighted atom (oxygen) is reduced.
To summarize:
- In reactions 1 and 2, the highlighted atom (oxygen) is reduced.
- In reaction 3, the highlighted atom (sulfur) is neither oxidized nor reduced.
- In reaction 4, the highlighted atom (oxygen) is reduced.
It's important to note that oxidation and reduction involve changes in the oxidation state of atoms, indicating the gain or loss of electrons. The analysis above is based on the change in oxidation state of the highlighted atom in each reaction.
for more questions on oxidized
https://brainly.com/question/13182308
#SPJ8
Chemistry Help Please! Worth a lot of points!
1. Joseph Priestly was the first scientist to be able to prepare pure oxygen gas. He did this by heating mercuric oxide as shown in the reaction below. Answer the question related to the reaction.
2HgO(s) = 2Hg(l) + O2(g)
a.What volume of oxygen gas would be produced at STP through the decomposition of 5.36 grams of HgO? It is not collected over water!
b. What volume of oxygen gas would be produced at 23o and 0.975 atm by the decomposition of 5.36 grams of HgO?
c. What volume of oxygen gas would be collected over water at 23oC and 0.975 atm by the decomposition of 5.36 grams of HgO?
2. How many grams of water will form through the reaction of 550 mL of 2.5 M HCl and an excess amount of Ca(OH)2?
Ca(OH)2(aq) + 2HCl(aq) = CaCl2(aq) + 2H2O(l)
a. How many grams of calcium chloride will form if 250 mL of 2.0 M Ca(OH)2 reacts with 350 mL of 2.5 M HCl? Make sure to determine the limiting reagent!
3. Sam conducted an experiment where he reacted 5.0 grams of magnesium with an excess amount of oxygen gas to form magnesium oxide (MgO). When he conducted this in the lab, he obtained 8.06 grams of magnesium oxide.
a. Write and balance the chemical reaction.
b. Use stoichiometry to predict how many grams of magnesium oxide should form.
c. What is the theoretical yield?
d. What is the actual yield?
e. Calculate the percent yield.
4. Na(s) + Br2(L) = NaBr(s) (not balanced)
b.If 3.05 grams of sodium react with 10.0 grams of diatomic bromine, what is the excess reagent AND how many moles of excess reagent are present after the reaction?
c. How many grams of sodium bromide are formed?
Answers
Answer:
A.
1. a. 11.28L O2
b. 24.3L O2
c. 19.83L O2
2. a. 5.6 grams CaCl2
3. a. 2Mg + O2 = 2MgO
b. 8.06 grams MgO
c. 8.06 grams MgO
d. 8.06 grams MgO
e. 100%
4. a. 2Na + Br2 = 2NaBr
b. Excess reagent: Bromine; 0.5 moles
c. 10.6 grams NaBr
For the reaction represented by the equation N2 + 3H, -> 2NH3, how many moles of nitrogen are required toproduce 18 mol of ammonia?
Answers
9moles of N2
Explanations:
Given the reaction represented by the equation:
\(N_2+3H_2\rightarrow2NH_3\)Given the following parameters
• Moles of ammonia = 18 moles
According to stoichiometry, 1 mole of nitrogen produces 2 moles of ammonia. Therefore the moles of nitrogen required is given as:
\(\begin{gathered} moles\text{ of }N_2=\frac{1mole\text{ of }N_2}{2\cancel{moles\text{ of }NH_3}}\times18\cancel{moles\text{ of }NH_3} \\ moles\text{ of }N_2=9moles \end{gathered}\)Hence the moles of nitrogen required to produce 18 mol of ammonia is 9moles
Based on your answer to part (e) above, in what region of the electromagnetic spectrum does this wavelength fall in (if in visible region then specify the color)?Part E asked If the wavelength on the electromagnetic spectrum is 450 nm, what is this wavelength in meters? answer was 4.5*10^-7
Answers
The question requires us to specify in which part of the electromagnetic spectrum the wavelength given (450 nm) falls.
Considering the electromagnetic spectrum scheme presented in the image below, we can affirm that decreasing the wavelength means increasing the energy (the smallest wavelength values shown, for example, fall in the gama ray section, which is a very energetic wave). Also, we can see that the visible region of the spectrum occurs between 380 and 750 nm:
Therefore, the wavelength given (450 nm or 4.50 x 10^-7 m) falls within the visible region of the spectrum and it refers to the blue color.

What do the symbols tell you about theconditions of the reaction shown to the right
Answers
Answer:
the arrow
Explanation:
this show reaction give product which always appear on right side
The air particles in the jar become smaller.
Answers
Why does an increased temperature cause a reaction to occur slower? A. The increased temperature makes the molecules more resistant to sucessful collision, they bounce off of each other more often. B. The increased kinetic energy causes the particles to move faster, causing more collisions. C. It does not. The increased temperature causes the reaction to occur more quickly. D. The increased potential energy in the particles means more energy is needed from the environment for the activation energy.
Answers
An increased temperature generally causes a reaction to occur faster rather than slower. Therefore option C is correct.
The increased temperature leads to a higher average kinetic energy of the molecules, which results in more frequent and energetic collisions between the reactant particles.
This increased collision frequency and energy facilitate the breaking of chemical bonds and the formation of new bonds, leading to an accelerated reaction rate.
When the temperature is raised, the kinetic energy of the molecules increases. This means that the individual molecules move faster and possess a greater amount of energy. As a result, the molecules collide more frequently and with higher energy, enhancing the likelihood of successful collisions that lead to a reaction.
In summary, an increased temperature leads to a faster reaction rate by increasing the average kinetic energy of the particles, causing more frequent and energetic collisions.
To know more about temperature-
brainly.com/question/15520591
#SPJ1
6 of 28
Attempt 2
If 7.66 g of CuNO, is dissolved in water to make a 0.140 M solution, what is the volume of the solution in milliliters?

Answers
The volume of a 0.140 M solution of Cu(NO3)2 that contains 7.66 g of the compound, volume of the solution is 292.9 mL.
To determine the volume of a 0.140 M solution of Cu(NO3)2 that contains 7.66 g of the compound, we can use the following formula:
Molarity = moles of solute / volume of solution in liters
First, we need to calculate the number of moles of Cu(NO3)2 in the given mass of the compound:
moles of Cu(NO3)2 = mass / molar mass
The molar mass of Cu(NO3)2 can be calculated by adding the atomic masses of copper, nitrogen, and six oxygen atoms:
1 x Cu = 63.55 g/mol
2 x N = 14.01 g/mol x 2 = 28.02 g/mol
6 x O = 15.99 g/mol x 6 = 95.94 g/mol
Molar mass of Cu(NO3)2 = 63.55 g/mol + 28.02 g/mol + 95.94 g/mol = 187.51 g/mol
Now, we can calculate the number of moles of Cu(NO3)2:
moles of Cu(NO3)2 = 7.66 g / 187.51 g/mol = 0.0409 moles
Finally, we can use the formula above to calculate the volume of the solution:
0.140 M = 0.0409 moles / volume of solution in liters
Volume of solution in liters = 0.0409 moles / 0.140 M = 0.2929 L
Converting to milliliters, we get:
Volume of solution in milliliters = 0.2929 L x 1000 mL/L = 292.9 mL
Therefore, the volume of the solution is 292.9 mL.
For more such questions on volume
https://brainly.com/question/28853889
#SPJ11
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
Answer:
AgCl = 0.0133 mol
Explanation:
The balanced chemical equation for the reaction between AgNO3 and CaCl2 is:
AgNO3 + CaCl2 → AgCl + Ca(NO3)2
From the equation, we can see that 1 mole of AgNO3 reacts with 1 mole of CaCl2 to produce 1 mole of AgCl. Therefore, we need to determine the number of moles of AgNO3 and CaCl2 in the given volumes of solutions and use the stoichiometric coefficients to calculate the number of moles of AgCl produced.
First, let's calculate the number of moles of AgNO3 in 63.57 mL of 1.327 M solution:
moles of AgNO3 = volume (in L) x concentration
moles of AgNO3 = 63.57 mL x 1 L/1000 mL x 1.327 mol/L
moles of AgNO3 = 0.0844 mol
Next, let's calculate the number of moles of CaCl2 in 41.87 mL of 0.317 M solution:
moles of CaCl2 = volume (in L) x concentration
moles of CaCl2 = 41.87 mL x 1 L/1000 mL x 0.317 mol/L
moles of CaCl2 = 0.0133 mol
Since we have more AgNO3 than CaCl2, CaCl2 is the limiting reagent. Therefore, the number of moles of AgCl produced is equal to the number of moles of CaCl2:
moles of AgCl = 0.0133 mol
what is a arieal animals?
Answers
in the following reaction, fe2o3 3co ---> 2fe 3co2, how many grams fe2o3 are needed to make 51.96 grams of co?
Answers
In order to make 51.96 grams of CO from Fe₂O₃ and 3CO, you would need to use 27.0 grams of Fe₂O₂.
The equation for the reaction between Fe₂O₃ and 3CO is Fe₂O₃ + 3CO → 2Fe + 3CO₂. In order for the reaction to take place, the moles of each reactant must be in a 1:3 ratio. Since you need 51.96 grams of CO, this means that the amount of Fe₂O₃needed is 27.0 grams. This is because 27.0 grams of Fe₂O₃ would contain 1 mole of Fe₂O₃ and 3 moles of CO, which is the 1:3 ratio needed for the reaction to take place.
The reaction between Fe₂O₃ and 3CO is an exothermic reaction. This means that when the reaction occurs, energy is released in the form of heat. This energy can be used to power various applications, such as the production of electricity. Furthermore, the reaction can be used to produce iron, which is a useful material in many industries.
Learn more about The reaction between Fe₂O₃:
https://brainly.com/question/1411474
#SPJ4
at a blood bank there are five problems with a labortory technician's work performance the first year of work
Answers
The performance of a laboratory technician in a blood bank is crucial as it directly impacts the quality of the blood products and patient safety. If there are five problems with a technician's work performance in the first year of work, it can have serious consequences for the blood bank's operations.
Some of the potential problems that may arise include:
Improper labeling of blood products: This can result in confusion and incorrect transfusions.
Mishandling of blood products: This can lead to contamination, spoilage, or improper storage, which can affect the quality of the blood products.
Failure to follow standard operating procedures: This can result in errors, deviations from protocols, and potential safety hazards.
Poor communication skills: This can result in misunderstandings, delays, and errors in documentation.
Inadequate training or knowledge: This can lead to mistakes, misinterpretation of test results, and failure to recognize potential problems.
for more such questions on laboratory
https://brainly.com/question/29482908
#SPJ11
7. One way to isolate metals from their ores is to react the metal oxide with carbon as shown in the
following reaction:
2MO(s) + C(s) -> 2M(s) + CO2(g)
If 34.49 g of a metal oxide reacted with excess carbon and 4.42 L of CO2 formed at 100°C and
1.50 atm, what is the identity of the metal?
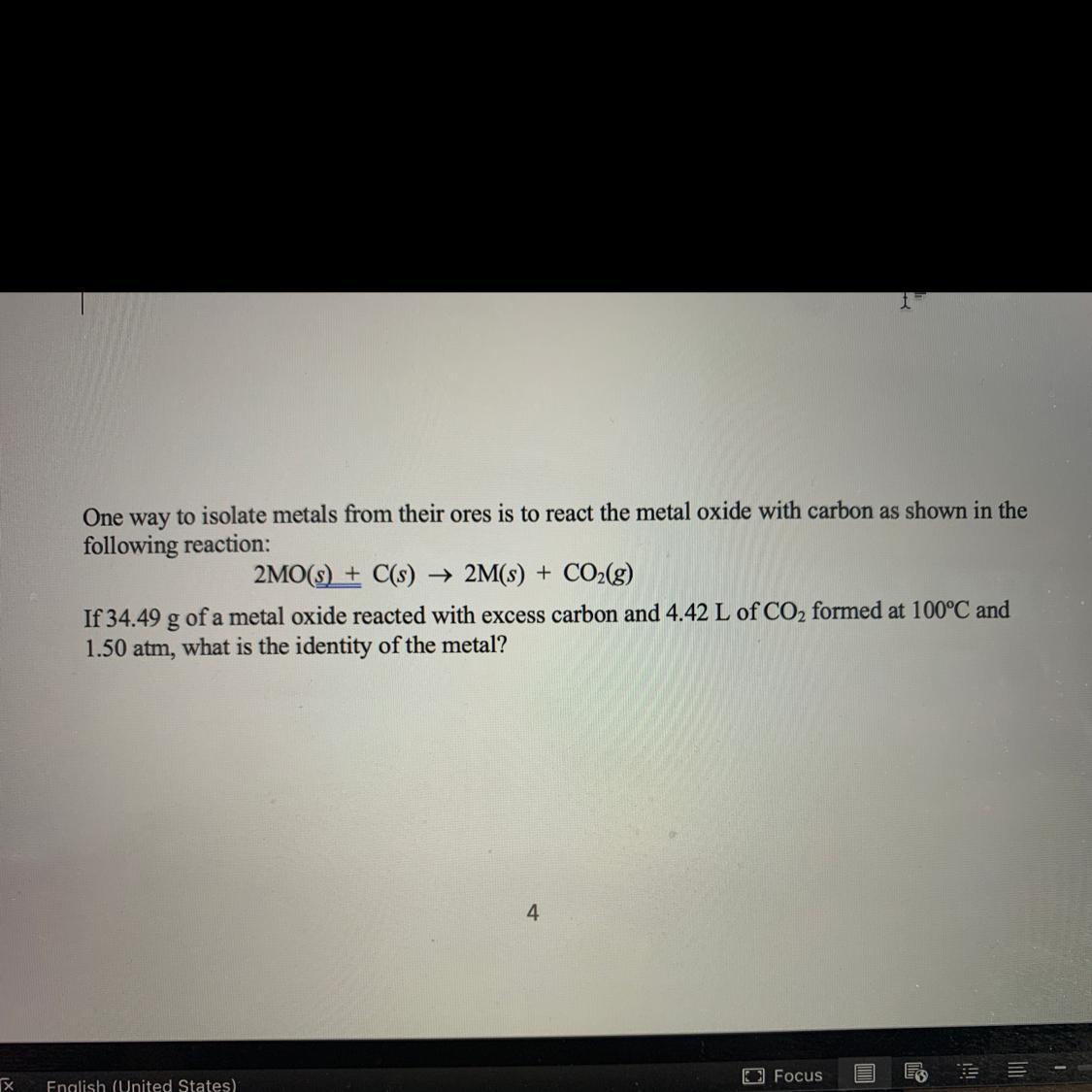
Answers
Metal oxides react with carbon and are reduced to the metal. The metal in the reaction above is copper.
The equation of the reaction is;
2MO(s) + C(s) -> 2M(s) + CO2(g)
We are told in the question that carbon is in excess so the metal oxide is the limiting reactant.
Number of moles of metal oxide = 34.49 g/M + 16
From PV = nRT
P = 1.50 atm
V = 4.42 L
T = 100°C + 273 = 373 K
n =?
R = 0.082 atmLK-1mol-1
n = PV/RT
n = 1.50 atm × 4.42 L/0.082 atmLK-1mol-1 × 373 K
n = 6.63/30.58
=0.217 moles
If 2 moles of metal oxide yields 1 mole of CO2
34.49 g/M + 16 yields 0.217 moles of CO2
34.49 g/M + 16 = 2 × 0.217
34.49 /M + 16 = 0.434
34.49 = 0.434(M + 16)
34.49 = 0.434M + 6.944
M = 34.49 - 6.944/0.434
M = 63.5
The metal is copper.
Learn more: https://brainly.com/question/22824409
A researcher wants to determine the success rate of a driver’s education program conducted in high schools in a particular state. Which method would assure random selection of a sample from the population? The researcher should select one high school in the state and survey all of the students in that school who are enrolled in the training program. The researcher should randomly select one high school in the state and survey a random batch of students who are enrolled in the training program. The researcher should randomly select a city in the state and survey all students in that city who are enrolled in the training program. The researcher should randomly select students from among all the students in the state who are enrolled in the training program.
Answers
The method that would assure random selection of a sample from the population is:
The researcher should randomly select students from among all the students in the state who are enrolled in the training program.
This method ensures that all students enrolled in the driver's education program in the state have an equal chance of being selected for the study. Random selection helps to eliminate bias and increases the likelihood that the sample is representative of the entire population.
The other options listed would not assure random selection of a sample from the population.
Selecting one high school or one city could introduce bias into the sample, as the success rate of the driver's education program may vary between different schools or cities.
Thus, selecting a random batch of students from one high school could also introduce bias into the sample, as the students selected may not be representative of all students enrolled in the driver's education program in the state.
For more details regarding Random selection, visit:
https://brainly.com/question/10678373
#SPJ1
The value of AG at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,
25 (s, rhombic) + 302 (g) → 2SO3 (g)
AG-370.4 kJ/mol.
+740.0
-740.8
-200,
kJ/mol.
+200.
Answers
The value of ΔG at 25 °C for the given reaction is: ΔG = -370.4 kJ/mol + 0 = -370.4 kJ/mol So, the correct answer is -370.4 kJ/mol
To determine the value of ΔG (Gibbs free energy) at 25 °C for the given reaction:
25 (s, rhombic) + 3/2 \(O_2\)(g) → \(2SO_3\)(g)
We can use the equation:
ΔG = ΔG° + RT ln(Q)
where:
ΔG is the standard Gibbs free energy change
ΔG° is the standard Gibbs free energy change under standard conditions
R is the gas constant (8.314 J/(mol·K) or 0.008314 kJ/(mol·K))
T is the temperature in Kelvin (25 °C = 298 K)
Q is the reaction quotient, which is the ratio of the concentrations of the products to the concentrations of the reactants at a given point during the reaction.
Given that ΔG° is -370.4 kJ/mol, we can plug the values into the equation:
ΔG = -370.4 kJ/mol + (0.008314 kJ/(mol·K) * 298 K) * ln(Q)
Now, we need to determine the value of Q. Since all reactants and products are in their standard states, Q = 1, as their concentrations are taken to be 1.
ΔG = -370.4 kJ/mol + (0.008314 kJ/(mol·K) * 298 K) * ln(1)
Since ln(1) = 0, the term (0.008314 kJ/(mol·K) * 298 K) * ln(1) becomes 0.
Therefore, the value of ΔG at 25 °C for the given reaction is:
ΔG = -370.4 kJ/mol + 0 = -370.4 kJ/mol
So, the correct answer is -370.4 kJ/mol.
For more such questions on reaction visit:
https://brainly.com/question/11231920
#SPj8
C2H4O2 IS THE EMPIRICAL FORMULA FOR GLUCOSE?
Answers
The correct empirical formula of the glucose molecule is \(CH_{2} O\).
What is the empirical formula?The empirical formula is the simplest formula of the compound. Let us note that the structural formula shows the arrangement of the atoms of the compound while the molecular formula shows the number of each of the atoms that is in the substance.
Now we know that the empirical formula determines the ratio of each of the atoms that are in a compound as such the formula that is shown can not be the empirical formula of glucose as we can see in the question that is above here.
Learn more about empirical formula:https://brainly.com/question/14044066
#SPJ1
A root is an example of a/an-
A: tissue
B: cell type
C:organ
D:organism
Answers
Answer: D an organism
Explanation:
the answer is C.
have a good day mate
How many moles in 2.33E25 molecules of NO?
0.0258 mol
3.87E20 mol
38.7 mol
2.58E48 mol
please show work
Answers
Answer:
0.0258 mol Answer .......
Write the acid-base reaction that occurs between HF and water . Identify the acid , base , conjugate acid, and conjugate base
Answers
The conjugate acid-base pairs are ( HF, F⁻) and ( H₂O, H₃O⁺ )
What is conjugate acid base pair?A conjugate acid-base pair, as defined by Bronsted-Lowry, consists of two compounds that are distinct only in that they contain a proton (H⁺). The addition of a proton to a base results in the formation of a conjugate acid, while the removal of a proton from an acid result in the formation of a conjugate base.
The reaction becomes:
HF + H₂O → H₃O⁺ + F⁻
The conjugate acid-base pairs are ( HF, F⁻) and ( H₂O, H₃O⁺ )
Here,
F⁻ to HF is conjugate acid.H₃O⁺ to H₂O is conjugate base.HF to F⁻ is conjugate base.H₂O to H₃O⁺ is conjugate acid.To know more about conjugate acid-base refer to:
https://brainly.com/question/22514615
#SPJ1
A coil of wire can become a temporary magnet if connected to a battery.
(true of false question btw)
Answers
Hope it helps you
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
glucose is a six carbon sugar. Albumin is a protein with 607 amino acids. the average molecular weight of a single amino acid is 135 g/mol. there is no reason to run these solutes at the 20 MWCO because
Answers
There is no reason to run these solutes at the 20 MWCO because they are both much smaller than the MWCO of the membrane.
The MWCO (molecular weight cut off) is the molecular weight of a solute at which it will be retained by a membrane during a process such as ultrafiltration or dialysis. If a solute has a molecular weight higher than the MWCO of a membrane, it will be retained and not pass through the membrane. If the molecular weight of a solute is lower than the MWCO, it will pass through the membrane.
In this case, glucose has a molecular weight of 180 g/mol (6 carbons x 12 g/mol per carbon + 6 oxygens x 16 g/mol per oxygen) and albumin has a molecular weight of approximately 81,942 g/mol (607 amino acids x 135 g/mol per amino acid). Both of these solutes have molecular weights that are much lower than 20,000 g/mol, which is a typical MWCO for ultrafiltration or dialysis membranes.
They would both easily pass through the membrane and be lost during the process. Instead, a membrane with a much lower MWCO would be needed if we wanted to retain these solutes during a process such as ultrafiltration or dialysis.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
the change of one or more substancss into other substance
Answers
Answer:
lol what? bruh you need to explain what your talking about im in an ap class i can help
Explanation:
Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M
Answers
Answer:
\(= \mathbf{C_3H_6O}\)
Explanation:
From the given information, since the molecular mass of the ion M+ is not given;
Let's assume M+ = 58.0423
So, by applying the 13th rule;
we will need to divide the mass by 13, after dividing it;
The quotient n = no. of carbon; &
The addition of the quotient (n) with the remainder r = no. of hydrogen.
So;
\(\dfrac{58}{13}= 4 \ remainder \ 6\)
So;
\(C_nH_{n+r} = C_4H_{4+6}\)
\(= C_4H_{10}\)
From the given information; we have oxygen present, so since the mass of oxygen = 16, we put oxygen in the molecular formula by removing \(CH_4\). Also, since the mass is an even number then Nitrogen is 0.
So, we have:
\(= \mathbf{C_3H_6O}\)
the normal human eye responds to visible light of wavelength range from about 390 to 710nm. Determine the frequency range of the human eye.
Answers
Explanation:
=>7.7 x10¹⁴ to 4.2 x10¹⁴Hz
