Sodium can be determined by flame emission spectrometry with a lithium internal standard. the emission intensities of standard solutions of nacl and an unknown containing a constant amount of licl were measured. all the intensities were corrected for background by subtracting the intensity of a blank.
ck, ppm intensity of k emission intensity of li emission
1 10 10
2 15.3 7.5
5 34.7 6.8
7.5 65.2 8.5
10 95.8 10
20 110.2 5.8
unknown 47.3 9.1
required:
a. plot the k emission intensity vs. the concentration of k, and determine the linearity from the regression statistics.
b. plot the ratio of the k intensity to the li intensity vs. the concentration of k, and compare the resulting linearity to that in part (a). why does the internal standard improve linearity?
c. calculate the concentration of k in the unknown.
Answers
a. To plot the k emission intensity vs. the concentration of k, we can use the given data for the standard solutions of NaCl.
The concentration of K can be expressed in parts per million (ppm) and the corresponding intensity values can be plotted on a graph. Using regression analysis, we can determine the linearity of the data. The resulting graph should show a linear relationship between concentration and intensity.
b. To plot the ratio of the k intensity to the li intensity vs. the concentration of k, we can divide the intensity of K by the intensity of Li for each standard solution and the unknown.
The resulting values can be plotted against the concentration of K. The linearity of this graph can also be determined using regression analysis. The internal standard improves linearity because it helps to correct for any variations in sample handling and instrument response, resulting in more accurate and precise measurements.
c. To calculate the concentration of K in the unknown, we can use the ratio of the intensity of K to Li and the calibration curve obtained from the standard solutions.
From the graph in part (b), we can determine the concentration of K in the unknown by finding its corresponding value on the x-axis. Alternatively, we can use the regression equation obtained from part (a) to calculate the concentration of K in the unknown based on its measured intensity.
To know more about emission intensity :
https://brainly.com/question/29036978
#SPJ11
Related Questions
1 point
Using the equation 4HCl(aq) + O2(g) + 2Cl2(g) + 2H₂O(g) +20kJ, if [HCI]=0.302,[0₂]=0.109,[Cl₂]=0.883,[H₂O]=0.166, find Keq.
23.7
7.5
Answers
Answer:
Therefore, the equilibrium constant (K) for the given reaction is 23.7.
Explanation:
To calculate the equilibrium constant (K), we need to use the law of mass action. The law of mass action states that the product of the concentrations of the products raised to their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients is equal to the equilibrium constant.
The balanced chemical equation is:
4HCl(aq) + O2(g) + 2Cl2(g) + 2H₂O(g) ⇌ 4ClH0.5(aq)
The stoichiometric coefficients indicate that the reaction involves a one-to-one ratio of reactants to products. Therefore, we can write:
K = [ClH0.5]^4 / [HCl]^4 [O2] [Cl2]^2 [H2O]^2
Substituting the given concentrations into the expression, we get:
K = [(0.883/2)^4] / [(0.302)^4 (0.109) (0.883)^2 (0.166)^2]
Simplifying the expression and calculating, we get:
K = 23.7
Therefore, the equilibrium constant (K) for the given reaction is 23.7.
given that the delta g value for a particular reaction is negative what can be said about relative portions of reactants and products when the reaction reaches equilibrium
Answers
When delta G value for a particular reaction is negative, then we can say that : there are fewer reactant molecules and then product molecules.
What is the characteristic of reaction if Delta G is negative?A negative ∆G means that reactants have more free energy than the products. Exergonic reactions are also called spontaneous reactions, as they can occur without addition of energy.
When used in a reaction, delta G is the difference in free energy between products and reactants. If this value is negative, then the reaction is spontaneous and if it is positive, then the reaction is non-spontaneous. If it is zero, then the reaction is at equilibrium.
To know more about delta G in reaction, refer
https://brainly.com/question/4506650
#SPJ4
what chest electrode is placed on the fifth intercostal space on the mid-clavicular line?
Answers
The chest electrode placed on the fifth intercostal space on the mid-clavicular line is known as the V4 electrode. V4 is part of the precordial electrodes used in a standard 12-lead electrocardiogram (ECG) to assess the electrical activity of the heart.
The placement of these electrodes is crucial for obtaining an accurate ECG, which helps healthcare professionals diagnose and monitor various cardiac conditions.
The V4 electrode is positioned on the left side of the chest in line with the middle of the clavicle (collarbone), at the level of the fifth intercostal space. To locate this space, one should palpate the ribcage and count down from the first rib until the fifth rib space is found. Proper electrode placement ensures that the ECG will accurately capture the electrical signals originating from the heart.
Other precordial electrodes, such as V1, V2, V3, V5, and V6, are also strategically placed on specific areas of the chest to obtain a comprehensive view of the heart's electrical activity. Collectively, the information from all 12 leads provides a detailed picture of the heart's functioning, assisting in the diagnosis of heart conditions and guiding treatment decisions.
In conclusion, the V4 electrode plays a vital role in electrocardiography, as its placement on the fifth intercostal space on the mid-clavicular line enables healthcare professionals to monitor the heart's electrical activity and make informed decisions about patient care.
to learn more about chest electrode, refer:-
https://brainly.com/question/31672512
#SPJ11
A metal can is able to withstand 3,800 kPa before it bursts. The gas in the can has a volume of 235 mL and the pressure is 110 kPa at 25°C. If the can is crushed to a volume of 8. 5 mL and the temperature does not change will it burst? What is the pressure of the gas in the can?
Answers
The pressure of a gas is directly proportional to its volume, according to Boyle's Law. this pressure is less than the maximum pressure that the can can withstand (3,800 kPa), the can will not burst.
Therefore, we can use the following equation to find the pressure of the gas in the can after it is crushed: P1V1 = P2V2 where P1 and V1 are the initial pressure and volume of the gas, and P2 and V2 are the final pressure and volume of the gas, respectively. Given that the initial volume (V1) of the gas in the can is 235 mL and the initial pressure (P1) is 110 kPa, we can substitute these values into the equation P1V1 = P2V2 110 kPa × 235 mL = P2 × 8.5 mL Solving for P2, we get: P2 = (110 kPa × 235 mL) / 8.5 mL P2 = 3,027 kPa Therefore, the pressure of the gas in the can after it is crushed to a volume of 8.5 mL is 3,027 kPa. Since this pressure is less than the maximum pressure that the can can withstand (3,800 kPa), the can will not burst.
learn more about pressure at :
https://brainly.com/question/12971272
#SPJ4
Atoms and molecules are particles that make upA.massB.MatterC.Substance
Answers
The properties of compounds depend on their structure.
When 2 or more atoms link up, they create a molecule. And we can say that a collection of molecules is called a compound.
Matter on Earth is in the form of solid, liquid, or gas. Solids, liquids, and gases are made of tiny particles called atoms and molecules.
Answer: B. Matter
If the AGº for ATP hydrolysis is -30 kJ/mol and the AG" for phosphoenolpyruvate hydrolysis is -62 kJ/mol, what is the AGº for the phosphorylation of ADP by phosphoenolpyruvate? -92 kJ/mol +31 kJ/mol +92 kJ/mol -62 kJ/mol -32 kJ/mol
Answers
The AGº for the phosphorylation of ADP by phosphoenolpyruvate is equal to 32 kJ/mol.
The AGº for the phosphorylation of ADP by phosphoenolpyruvate can be calculated using the equation:
AGº = AGº (ATP hydrolysis) - AGº (phosphoenolpyruvate hydrolysis)
Given that AGº (ATP hydrolysis) = -30 kJ/mol and AGº (phosphoenolpyruvate hydrolysis) = -62 kJ/mol, then:
AGº = -30 kJ/mol - (-62 kJ/mol) = 32 kJ/mol
NADH: An increase in the concentration of NADH can inhibit PDH, as it competes with pyruvate for binding to the active site of the enzyme.
Acetyl-CoA: An increase in the concentration of acetyl-CoA can also inhibit PDH, as it acts as an allosteric inhibitor
Therefore, the AGº for the phosphorylation of ADP by phosphoenolpyruvate is equal to 32 kJ/mol.
Learn more about concentration here
https://brainly.com/question/10725862
#SPJ4
which of the following would form an ionic bond?
a.strontium and oxygen
b. sulfur and oxygen
Answers
Sodium and magnesium are both found in the third period of the periodic table. Which of these elements has the larger atomic radius, and what best explains the difference in their atomic radii?
Answers
The element of Sodium in the periodic table has a large atomic radius than magnesium due to less atomic attraction on the last electron.
What is the atomic radius?The atomic radii are given as the distance between the last electron in the shell to the center of the nucleus.
In the periodic table, on moving from the left to right there has been a decrease in the atomic radius of the atom with the addition of an electron in the same shell forming an increase in the nuclear attraction faced by the last electron.
Thus, Sodium has a large atomic radius than magnesium due to the presence of a less nuclear attraction over the last electron.
Learn more about atomic radii, here:
https://brainly.com/question/14086621
#SPJ4
Find the theoretical oxygen demand for the following
solutions:
a. 200 mg/L of octanol,
CH3(CH2)7OH
b. 90 mg/L of acetone, C3H6O
Please explain steps
Answers
To find the theoretical oxygen demand for the given solutions, we need to calculate the amount of oxygen required to completely oxidize the organic compounds present in each solution.
This can be determined by using the stoichiometry of the balanced chemical reactions representing the oxidation of the organic compounds.
a. Octanol (CH3(CH2)7OH)
The balanced chemical equation for the oxidation of octanol is as follows:
2C8H18 + 25O2 -> 16CO2 + 18H2O
From the balanced equation, we can see that for every 2 moles of octanol (C8H18), 25 moles of oxygen (O2) are required.
Given that the concentration of octanol is 200 mg/L, we can convert it to moles per liter:
200 mg/L * (1 g / 1000 mg) * (1 mol / molar mass of octanol)
Next, we can calculate the theoretical oxygen demand:
Oxygen demand = (moles of octanol) * (25 moles of oxygen / 2 moles of octanol)
b. Acetone (C3H6O)
The balanced chemical equation for the oxidation of acetone is as follows:
C3H6O + 4O2 -> 3CO2 + 3H2O
From the balanced equation, we can see that for every 1 mole of acetone (C3H6O), 4 moles of oxygen (O2) are required.
Given that the concentration of acetone is 90 mg/L, we can convert it to moles per liter:
90 mg/L * (1 g / 1000 mg) * (1 mol / molar mass of acetone)
To learn more about compounds
https://brainly.com/question/26556885
#SPJ11
Figure out a) (ii). TEST PREP HELLLPPPPP

Answers
Aii. The percentage abundance of each isotopes are:
Abundance of ¹⁰B is 19%Abundance of ¹¹B is 81%Ai. What is isotope of element?Isotopes of an element is defined as the atoms of the element having the same number of protons but different neutron number
Aii. How do i determine the percentage abundance?The percentage abundance of each isotope can be obtain as illustrated below:
Mass of 1st isotope, ¹⁰B = 10 amuMass of 2nd isotope, ¹¹B = 11.009 amuAverage atomic mass of boron = 10.81 amuAbundance of 1st isotope, ¹⁰B (1st%) = A =?Abundance of 2nd isotope, ¹¹B (2nd%)= 100 - A =?Average atomic mass = [(Mass of 1st × 1st%) / 100] + [(Mass of 2nd × 2nd%) / 100]
10.81 = [(10 × A) / 100] + [(11 × (100 - A) / 100]
10.81 = 0.1A + 11 - 0.11A
Collect like terms
10.81 - 11 = 0.1A - 0.11A
-0.19 = -0.01A
Divide both sides by -0.01
A = -0.19 / -0.01
Abundance of 1st isotope, ¹⁰B = 19%
Abundance of 2nd isotope, ¹¹B = 100 - A
Abundance of 2nd isotope, ¹¹B = 100 - 19
Abundance of 2nd isotope, ¹¹B = 81%
Learn more about percentage abundance of isotope:
https://brainly.com/question/11026801
#SPJ1
The electron shell model of an atom has three main components the energy shell, the subshell, and the orbital. Arrange these components from the lowest to highest maximum capacity to hold electrons. The component that can hold the greatest number of electrons should be at the top.

Answers
The selection rules of quantum mechanics allow finding the result for the order of the atomic levels are:
number higher electrons -- lower number electrons
Principal > Orbital > Magnetic
n > l > \(m_l\)
The solution of the Schrodinger equation of quantum mechanics results in the energy of the in electrons in an atom that has spherical symmetry and has three constants that are related.
The constants are called quantum numbers and are: The main, the secondary or orbital and magnetic.
The principal quantum number (n) can have values from 0 to infinity
The orbital quantum number (l) can have a value from 0 to n -1, it is customary to write this number with letters
number symbol
0 s
1 p
2 d
3 f
The magnetic quantum number (\(m_l\) ) can have values from -l to l
Apart from this number there is a fourth quantum number called spin magnetic quantum number (\(m_s\)) and it can have only two values ½ and -½,
These numbers that are allowed in quantum mechanics are called select rules. We can see that in each main number (n) there can be several orbital numbers (l) and within each orbital number there can be several magnetic numbers and within each of them there is
The order of the levels from highest to lowest number of electrons are:
Spin. Lowest
Mmagnetic
Orbital
Principal Highest
In conclusion using the selection rules of quantum mechanics we can find the result for the order of the atomic levels are:
Number higher electrons -- Lower number electrons
Principal > Orbital > Magnetic
n > l > \(m_l\)
Learn more here: brainly.com/question/11855107
predict the products of the following elimination reaction, and draw the major product formed. make sure to consider the stereochemistry of the reaction.
Answers
1) Draw the reactants in their anti-periplanar conformation.
2) Eliminate one of the hydrogens, forming a double bond between the two remaining atoms.
3) Consider the stereochemistry of the reaction to determine if the double bond is in a cis or trans conformation.
4) The resulting product is the major product formed from the elimination reaction.
The reaction you have provided is an elimination reaction, in which an alkene product is formed. The products of this reaction will depend on the reactants provided and the conditions used for the reaction. In particular, the major product of this reaction will be the one that follows the Zaitsev’s rule. When considering the stereochemistry of the reaction, the anti-periplanar conformation of the reactants must be taken into account.
The major product of the following elimination reaction will be an alkene with the double bond formed in the anti-periplanar conformation that follows Zaitsev's rule.
Learn more about Zaitsev’s rule
brainly.com/question/29675824
#SPJ11
What is this help ASAP
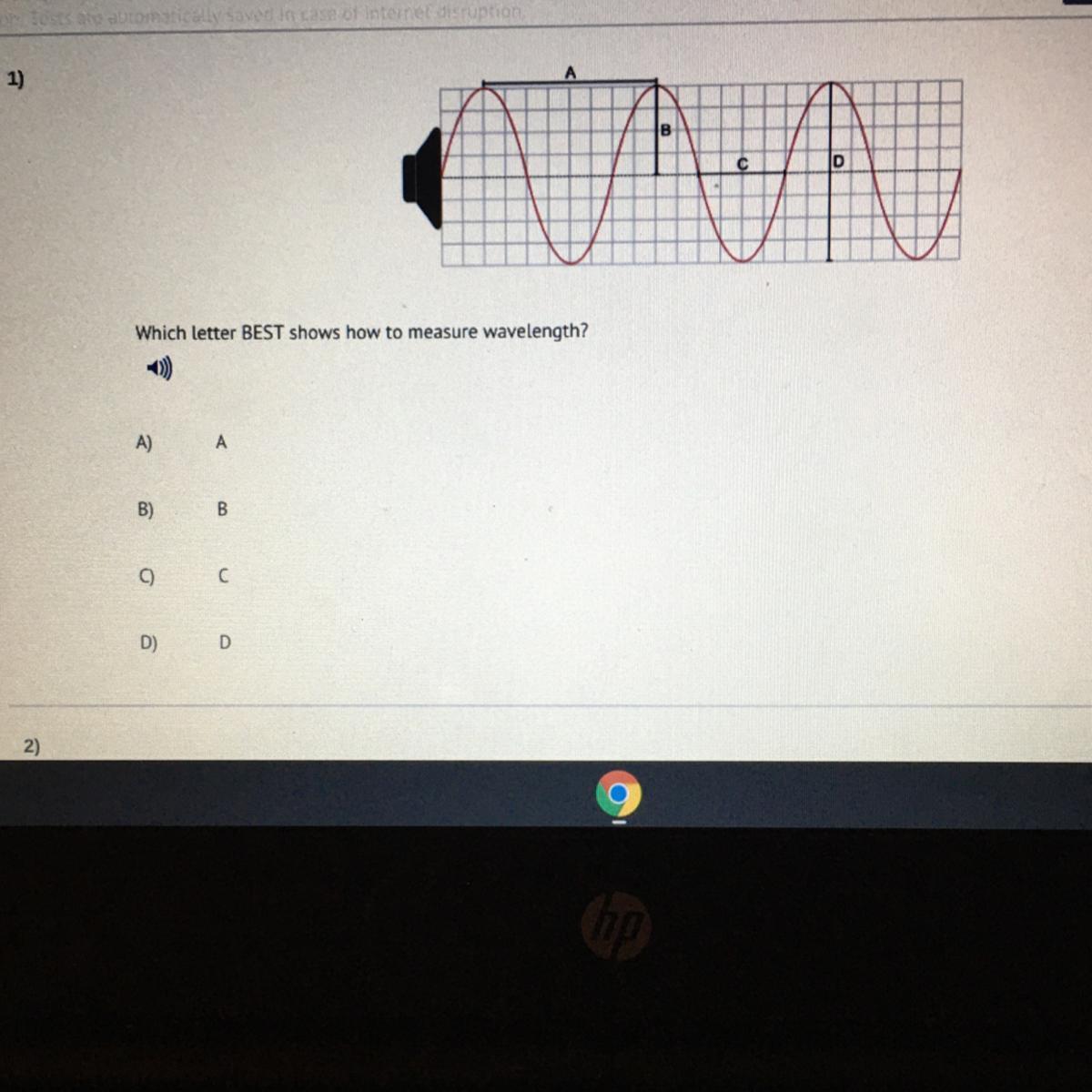
Answers
Answer:
A, im gonna say. Hopefully this helps!
Explanation:
Length is horizontal, and A is horizontal too.
what are the essential ingredients of a symmetric cipher?
Answers
A symmetric cipher typically has the following essential ingredients: key, plaintext, encryption algorithm, decryption algorithm.
A key: A secret value shared between the sender and the receiver that is used to encrypt and decrypt the message.
A plaintext: The original, unencrypted message.
An encryption algorithm: A mathematical function that takes the plaintext and the key and produces an encrypted message (ciphertext).
A decryption algorithm: A mathematical function that takes the ciphertext and the key and produces the original plaintext.
A method for securely sharing the key between the sender and receiver, as the security of a symmetric cipher relies on the secrecy of the key.
Note: The exact ingredients may vary depending on the specific symmetric cipher being used.
Learn more about encryption algorithm here:
https://brainly.com/question/29533438
#SPJ4
4Pb3(PO4)4
Ph=
S=
O=
Answers
Answer:
BF3
Explanation:
How many liters of oxygen are needed to react completely with 81.0 g of aluminum at STP?
A.) 25.2 L
B.) 72.0L
C.) 50.4 L
D.) 67.2 L
Answers
Answer:
Volume O₂ at STP = 50.4 Liters
Explanation:
4Al(s) + 3O₂(g) => 2Al₂O₃(s) at STP conditions
81g Al(s) = 81g/27g/mole = 3mole Al
moles O₂ consumed = 4/3(3)moles O₂ = 2.25 moles O₂ consumed
Volume O₂ at STP = 2.25moles x 22.4L/mole = 50.4 Liters O₂
Al= 108g have Vo2= 67.2L
Al= 81.0g have Vo2= ?
What you need to do is:
67.2 multiply with 81.0 and divided by 108 you will see the answer which is 50.4 L
The homogeneity of the chloride level in a water sample from a lake was tested by analyzing portions drawn from the top and from near the bottom of the lake, with the following results
Top (ppm Cl)
Bottom (ppm Cl)
26.30
26.22
26.43
26.32
26.28
26.20
26.19
26.11
26.49
26.42
Apply the t-test at the 95% confidence level to determine if the chloride level from the top of the lake is different from that at the bottom.
Now use the paired t-test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level.
Why is a different conclusion drawn from using the paired t- test than from just pooling the data and using the normal t- test for differences in means?
Answers
The paired t-test yields a different conclusion than the normal t-test because it accounts for the paired nature of the data, comparing the measurements taken at the top and bottom of the lake separately.
In this scenario, the paired t-test is appropriate because it analyzes the data as pairs, considering the chloride levels measured at the top and bottom of the lake for each sample. By comparing the differences within each pair, the paired t-test determines whether there is a significant difference between the chloride levels at the top and bottom of the lake.
Using the paired t-test, the differences between the paired observations are calculated, and the null hypothesis assumes that the mean difference is zero (no significant difference between the top and bottom chloride levels). The test then determines whether the observed differences are statistically significant at a chosen confidence level, in this case, 95%.
The normal t-test for differences in means, on the other hand, would treat the top and bottom chloride levels as separate and unrelated groups, disregarding their paired nature. By pooling the data and conducting a standard t-test, the analysis assumes that the two sets of measurements are independent, which may not be appropriate in this case. This can lead to a different conclusion compared to the paired t-test.
The different conclusion drawn from using the paired t-test compared to pooling the data and using the normal t-test is due to the consideration of the paired nature of the measurements. The paired t-test takes into account the potential correlation or connection between the measurements taken at the same location (top and bottom of the lake) and assesses the differences within each pair.
Pooling the data and using the normal t-test treats the measurements as independent, disregarding the pairing. This can result in a loss of valuable information and may lead to an inaccurate conclusion. The paired t-test is more appropriate when dealing with dependent or related measurements, ensuring a more accurate assessment of the differences between the top and bottom chloride levels.
Learn more about paired t-test
brainly.com/question/32245864
#SPJ11
2. Why is soil considered limited?
Answers
Answer: he natural area of productive soils is limited – it is under increasing pressure of intensification and competing uses for cropping, forestry, pasture / rangeland and urbanization, and to satisfy demands of the growing population for food and energy production and raw materials extraction
sodium atoms emit light with a wavelength of 330 nm when an electron moves from a 4p orbital to a 3s orbital. what is the energy difference between the orbitals in kj/mol
Answers
The energy difference between the orbitals can be calculated from bohr's model.
According to the bohr's model, the electrons are in continuous motion round the nucleus closed orbits of definite energy level. as the distance of the cell increases from the nucleus ,energy level of cell increases..as long as electron occupy a definite energy level, it doesn't radiate out energy. The emission of energy occurs only when electron jumps from one level to other.
E= h c/ λ
Sodium atom emit light with a wavelength of 330nm when an electron moves from a 4p orbital to a 3s orbital.
λ=330nm
h=6.625 .10-34 (Planck's constant)
c= 3.0 .108m/s-1
putting the values in the expression of Energy we get the energy difference.
To learn more about Bohr's model please visit:
https://brainly.com/question/18002213
$SPJ4
5 points
14. What is the molality of a solution with 98.0 grams KOH in 425 grams
H20?*
A. 0.412 molal
B. 4.12 molal
C. 41.2 molal
D. 231 molal
Answers
Answer:
C 41.2
Explanation:
I took the test ez
brainliest and 5 star pls
25cm3 of 0.2mol/dm3 barium hydroxide solution reacted with 22.8cm3 hydrochloric acid. Calculate the concentration of the hydrochloric acid in mol/dm3. Ba(OH)2 + 2HCl BaCl2 + 2H2O
Answers
Explanation:
1 mol of Ba(OH)2 = 2 moles of HCl

HELP ME, MY LAST QUESTION WRONG
Which graph shows acceleration?
Which graph shows acceleration?
A graph of position (meters) versus time (seconds) has a straight line running from 0 seconds 0 meters upward.
A graph of position (meters) versus time (seconds) has a concave line running from 0 seconds 0 meters upward.
A graph of position (meters) versus time (seconds) has a straight line running from 0 seconds positive number of meters downward to some later time 0 meters.
Answers
Answer: Graph B (Concave)
Explanation:
Acceleration increases or decreases over time, a concave line would show that transformation rather it being a straight line.
how do I balance this?

Answers
Answer:
4NaHCO3---->2Na2CO3+2CO2+2H2O
what is the concentration, in m/v percent, of a solution prepared from 50. g nacl and 2.5 l of water?
Answers
The concentration, in m/v percent, of a solution prepared from 50. g nacl and 2.5 l of water is 2 %.
The mass/volume percent is the ratio of a solution's total volume to the mass of the solute that makes up that solution. Since this type of concentration has been expressed as a percentage, the given proportion should be multiplied by 100.
The calculation if concentration is shown as:
It can be calculate as follows:
% NaCl = Mass of NaCl / Total mass × 100 %
% NaCl = 50 gram / 2500 × 100 %
% NaCl = 2 %.
As a result, the solution made from 50 g of nacl and 2.5 l of water will have a 2% concentration.
To know more about concentration
brainly.com/question/10695134
#SPJ4
explain why rain is normally slightly acidic in a non-polluted atmosphere. in your own words define acid rain.
Answers
In addition to carbon dioxide, water also includes other gases from the atmosphere. When the carbon dioxide dissolves, it produces carbonic acid, giving regular rain a pH of 5.6 on the pH scale.
Describe acid rain?
Acid rain, also known as acid deposition, is any sort of precipitation that falls to the earth from the sky in wet or dry forms and contains acidic materials like sulfuric or nitric acid. This might include things like acidic dust, rain, snow, fog, and hail.
Why Does Acid Rain Occur?When sulfur dioxide (SO2) and nitrogen oxides (NOX) are released into the atmosphere and carried by wind and air currents, acid rain is the outcome. Nitric and sulfuric acids are created when the SO2 and NOX combine with water, oxygen, and other substances. Then, before hitting the ground, they combine with water and other substances.
To know know more about acid deposition?
https://brainly.com/question/7669303
#SPJ4
What is the mass, in grams, of 289.1 mol of uranium?
Answers
The mass in grams of uranium is 820.255g
Mass is the any substances that would be calculated by using their moles
Here given data is
uranium in 1 mol = 289.1 mol
And the formula is
n = W/V
W = required mass = ?
M = molar mass of uranium = 238.05g/mol
n = moles of uranium = 289.1 mol
Then putting this value
W = 289.1 mol×238.05g/mol
W = 820.255g
The mass, in grams of 289.1 mol of uranium is 820.255g
Know more about uranium
https://brainly.com/question/14048980
#SPJ1
A positively charged rod is brought near a
charged electroscope. As a result of doing
this, the electroscope leaves move closer
to each other. What is the charge on the
electroscope?
Answers
When a positively charged rod is brought near a charged electroscope, the electroscope leaves move closer to each other, indicating that the electroscope has a negative charge.
When a positively charged rod is brought near a charged electroscope, the positively charged rod polarizes the charges in the electroscope. Initially, the electroscope may have a neutral charge, meaning the number of positive and negative charges within it are balanced. However, when the positively charged rod is brought close to the electroscope, it repels the positive charges in the electroscope and attracts the negative charges.
As a result, the positive charges within the electroscope are pushed away from the rod, while the negative charges are attracted toward it. This redistribution of charges creates a separation of charge within the electroscope.
Since the positive charges are repelled and move away from the leaves of the electroscope, the negatively charged leaves experience a net attractive force. This force pulls the leaves closer together, causing them to move toward each other.
Therefore, the electroscope acquires a negative charge as a result of the positively charged rod being brought near it.
Learn more about electroscopes at https://brainly.com/question/13707421
#SPJ11
i need help asap !!!!!!

Answers
The trend of atomic radius refers to the predictable pattern of the size of atoms as one moves across or down the periodic table of elements.
What is the trend of atomic radius?Moving across a period (from left to right), the atomic radius of an element generally decreases. This is because the number of protons and electrons in the atom increases, leading to a stronger attractive force between the positively charged nucleus and negatively charged electrons. The increased pull causes the electron cloud to be pulled in closer to the nucleus, making the atomic radius smaller.
Moving down a group (from top to bottom), the atomic radius generally increases. This is because each successive element has one additional energy level than the element above it, and the electrons in these energy levels are farther from the nucleus. The increase in distance between the outermost electrons and the nucleus results in an increase in the atomic radius.
Therefore, the general trend of atomic radius is that it decreases from left to right across a period and increases from top to bottom down a group.
The following would have a larger atomic radius in the pair;
1) Ge
2) Fe
3) Kr
4) Sr
5) Na
6) I
Learn more about atomic radius:https://brainly.com/question/13607061
#SPJ1
based on the h and s values for a given chemical reaction, it is possible to predict whether the reaction is spontaneous or not at various temperatures. which one of the following statements has the most correct answers? question 38 options: (a) if h and s are both positive, the reaction will always be spontaneous (b) if h and s are both positive, the reaction will be spontaneous at a high enough temperature (c) if h is negative and s is positive, the reaction will always be spontaneous (d) if h and s are both negative, the reaction will always be spontaneous (e) both (b) and (c) g
Answers
The correct answer is (b) if h and s are both positive, the reaction will be spontaneous at a high enough temperature.
The spontaneity of a chemical reaction is determined by the change in Gibbs free energy (ΔG) of the system. The relationship between enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) is given by the equation:
ΔG = ΔH - TΔS,
where T represents the temperature in Kelvin.
For a spontaneous reaction, ΔG must be negative. From the equation, we can observe that if both ΔH and ΔS are positive, the reaction can still be spontaneous if the temperature is high enough. As the temperature increases, the negative term TΔS becomes more significant and can overcome the positive term ΔH, resulting in a negative ΔG.
Therefore, option (b) is the most correct statement, stating that if both h and s are positive, the reaction will be spontaneous at a high enough temperature.
Learn more about spontaneous reaction
brainly.com/question/14312067
#SPJ4
when a more complex compound breaks down into two simpler ones, it is known as a _______ reaction.
Answers
When a more complex compound breaks down into two or more simpler compounds, it is known as a decomposition reaction.
What do you mean by complex compound?
In chemistry, a complex compound is a substance that consists of two or more molecules or ions joined together by coordinate covalent bonds. These bonds are formed when one atom donates a pair of electrons to another atom that has an empty orbital. The atom that donates the electron pair is called the donor atom, and the atom that accepts the electron pair is called the acceptor atom.
When a more complex compound breaks down into two or more simpler compounds, it is known as a decomposition reaction. In other words, decomposition reactions involve the breaking down of a larger molecule into smaller molecules or atoms. This type of reaction is the opposite of a synthesis reaction, which involves the combining of two or more simpler compounds to form a more complex compound.
A decomposition reaction can occur through various mechanisms, including thermal decomposition, electrolysis, photolysis, and catalytic decomposition. The specific mechanism depends on the nature of the reactant and the conditions under which the reaction takes place.
Thermal decomposition reactions occur when a substance is heated to a temperature high enough to break its chemical bonds. For example, calcium carbonate decomposes into calcium oxide and carbon dioxide gas when heated:
CaCO3 (s) → CaO (s) + CO2 (g)
Learn more about decomposition reaction click here:
https://brainly.com/question/27300160
#SPJ1