Answers
Related Questions
identify reduction and oxidation process from below reactions.. al 3 (aq) mg (s) ------>> al (s) mg 2 ag fe 2 ------>>> ag (s) fe 3
Answers
The process of oxidation occurs when a reactant loses electrons during a reaction.
What is Is Al3+ 3e Al oxidation or reduction?Al3++3e Al is a properly balanced oxidation process, according to statement I.Silver has an oxidation number of 1, while aluminum has zero. Al and Silver's oxidation numbers are +3 and 0 on the product side, respectively. Al has a higher oxidation number. It is therefore oxidized and acts as a reducing agent.Statement I: The oxidation reaction of Al 3+ + 3e Al is properly balanced.In light of Statement II: Al 3+ +3e − →AlAl effectively exemplifies both mass and charge conservation.On both sides of the equation, the total number of charges is equal.Each side of the equation has the same total mass.It is true of both claims.Al 3+ +3e − →Al.
To Learn more about oxidation refer to:
https://brainly.com/question/25886015
#SPJ4
solid calcium reacts rigorously with liquid water to form aqueous calcium hydroxide and hydrogen gas. enter a balanced equation for the reaction.
Answers
Solid calcium reacts rigorously with liquid water to form aqueous calcium hydroxide and hydrogen gas. The balanced equation can be given as
CaH\(_2\) +2H\(_2\)O → Ca (OH)\(_2\) + 2H\(_2\)
What is chemical reaction?A chemical reaction is indeed a procedure that causes one group of chemical components to change chemically into another. Chemical bonds that link atoms are formed.
This is broken by traditional chemical processes, which only affect the locations of electrons and do not affect the nuclei of the atoms. Solid calcium reacts rigorously with liquid water to form aqueous calcium hydroxide and hydrogen gas. The balanced equation can be given as
CaH\(_2\) +2H\(_2\)O → Ca (OH)\(_2\) + 2H\(_2\)
Therefore, the balanced equation can be given as CaH\(_2\) +2H\(_2\)O → Ca (OH)\(_2\) + 2H\(_2\).
To know more about chemical reaction, here:
https://brainly.com/question/3461108
#SPJ1
Describe how advances in atomic theory led to the use of atomic energy.
Answers
Answer: Atomic theory established that all matter is made of tiny particles, a discovery that led to amazing scientific breakthroughs in areas from modern chemistry to nuclear energy
Explanation:
* GIVING BRAINLIEST*
3Ca+2 FeCl3 -> 3CaCl2 + 2Fe
Calcium metal + Iron Chloride -> Calcium Chloride + Iron metal
Identify the reason that atoms react with each other.
Answers
i think its double replacement if i'm not mistaken
During a laboratory experiment, a 3. 81-gram sample of NaHCO3 was thermally decomposed. In this experiment, carbon dioxide and water vapors escape and are combined to form carbonic acid. After decomposition, the sample weighed 2. 86 grams. Calculate the percentage yield of carbonic acid for the reaction. Describe the calculation process in detail. (10 points)
NaHCO3 → Na2CO3 + H2CO3
Answers
During a laboratory experiment, a 3. 81-gram sample of \(NaHCO$_3$\) was thermally decomposed. In this experiment, carbon dioxide and water vapors escape and are combined to form carbonic acid. Percentage yield ≈ 34.59%
The calculation of the percentage yield of carbonic acid \((H$_2$CO$_3$)\)
1. Determine the moles of \(NaHCO$_3$\):
Moles of \(NaHCO$_3$\) = Mass of \(NaHCO$_3$\) / Molar mass of \(NaHCO$_3$\)
Moles of \(NaHCO$_3$\) = 3.81 g / 84.01 g/mol
Moles of \(NaHCO$_3$ $\approx$ 0.04539 mol\)
2. Use stoichiometry to find the moles of \(H$_2$CO$_3$\) :
From the balanced equation, we can see that the molar ratio between \(NaHCO$_3$ and H$_2$CO$_3$\) is 1:1.
\(Moles of H$_2$CO$_3$\) = \(Moles of NaHCO$_3$\)
3. Calculate the theoretical yield of \(H$_2$CO$_3$\) :
Theoretical yield of \(H$_2$CO$_3$\) = \(Moles of H$_2$CO$_3$ $\times$ Molar mass of H$_2$CO$_3$\)
Theoretical yield of \(_2$CO$_3$ $\approx$ 0.04539 mol $\times$ 62.03 g/mol\)
4. Calculate the percentage yield:
Percentage yield = (Actual yield / Theoretical yield) $\times$ 100%
Actual yield = Initial mass of \(NaHCO$_3$\) – Final mass after decomposition
Actual yield = 3.81 g – 2.86 g
Percentage yield = (Actual yield / Theoretical yield) x 100%
Percentage yield = (0.95 g / (0.04539 mol x 62.03 g/mol)) x 100%
Percentage yield ≈ 34.59%
The resulting value is the percentage yield of carbonic acid for the reaction.
Learn more about percentage yield here:
https://brainly.com/question/30139773
#SPJ11
QUESTION 7 What is the pH of water? O pH12 O pH9 O pH7 O pH5 QUESTION 8 What is the pH when fish die from pollution? O pH12 O pH9 O pH7 O pH4 QUESTION 9 A solution with a pH less than 7 is basic. O True O False
Answers
7. The pH of water is pH7.
The pH scale measures the acidity or alkalinity of a substance. It ranges from 0 to 14, with pH7 considered neutral. Water has a pH of 7, indicating that it is neither acidic nor basic. It is important to note that the pH of pure water can vary slightly due to the presence of dissolved gases and minerals, but it generally remains close to pH7.
8. When fish die from pollution, the pH is typically around pH4.
Pollution can introduce harmful substances into water bodies, leading to a decrease in pH. Acidic pollutants, such as sulfur dioxide and nitrogen oxides, can cause the pH of water to drop significantly. When fish are exposed to highly acidic water, their physiological processes are disrupted, and they may die as a result. A pH of around pH4 is considered highly acidic and can be detrimental to aquatic life.
9. A solution with a pH less than 7 is acidic.
This statement is false. A solution with a pH less than 7 is actually considered acidic, not basic. The pH scale ranges from 0 to 14, with pH7 being neutral. Solutions with a pH below 7 are acidic, indicating a higher concentration of hydrogen ions (H+) in the solution. On the other hand, solutions with a pH above 7 are basic or alkaline, indicating a higher concentration of hydroxide ions (OH-) in the solution.
To know more about Pollutants visit-
brainly.com/question/29594757
#SPJ11
write a net ionic equation for the overall reaction that occurs when aqueous solutions of sodium hydroxide and oxalic acid
Answers
A net ionic equation for the overall reaction that occurs when aqueous solutions of sodium hydroxide and oxalic acid is :
H₂C₂O₄ + 2OH⁻ ----> C₂O₄²⁻ + 2H₂O
The balance molecular equation is given as :
2NaOH (aq) + H₂C₂O₄ (aq) -----> Na₂C₂O₄ (aq) + 2H₂O (l)
now, the complete ionic equation is given as :
H₂C₂O₄ + 2Na⁺ + 2OH⁻ ----> 2Na⁺ + C₂O₄²⁻ + 2H₂O
cancel out the spectators ion the spectators ions are those which are on both side the reactant side and the product side : the net ionic equation is given as :
H₂C₂O₄ + 2OH⁻ ----> C₂O₄²⁻ + 2H₂O
To learn more about net ionic equation here
https://brainly.com/question/14963790
#SPJ4
How are the mass of the products and the mass of the reactants related?
Answers
Answer: They are the same
Explanation:
According to the law of conservation of mass, in a chemical reaction, the amount of matter in the reactants is the same as that of the products. Matter is neither created nor destroyed, thus the mass would, in turn, remain the same.
Which is the best description of a molecule?
Answers
Answer:
A molecule is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound. Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms.
Answer:
C
Explanation:
Calculate the expected pH values of the buffer systems from the experiments (a, b, c, d) using the Henderson-HAsselbach equation, pH= pKa + log([A-]/[HA]). Use for pKa values carbonic acid = 6.37 and acetic acid=4.75
for A 5 ml 0.1 M CH3COOH + 5 mL 0.1 M CH3COO-NA+ pH 4.1 after addition of 0.5mL 0.1 M HCL pH is 4.2 after addition of 0.5 mL 0.1 M NaOH pH is 4.1
for B 1mL M CH3COOH + 10 mL 0.1 M CH3COO-Na+ pH is 5.2 after addition of 0.5 mL 0.1 M HCL pH is 5.1 after addition of 0.5 mL 0.1 NaOH pH is 6.0
for C 5mL 0.1 M H2CO3 + 5mL 0.1 M NaHCO3 pH is 9.2 after addition of 0.5 mL 0.1 M HCL pH is 8.9 after addition of 0.5 mL 0.1 M NaOH pH is 9.5
for D 1mL 0.1 M H2CO3 + 10mL 0.1 M NaHCO3 pH is 8.6 after addition of 0.5 mL o.1 M HCl pH is 7.5 after addition of 0.5 mL 0.1 M NaOH pH is 9.0
Answers
The expected pH values of the buffer systems from the experiments are: A: 4.75 ; B: 4.25 ; C: 6.37 ; D: 5.77.
To calculate the expected pH values of the buffer systems from the experiments (a, b, c, d) using the Henderson-Hasselbalch equation, pH= pKa + log([A⁻]/[HA]), we need to first determine the [A⁻]/[HA] ratio for each experiment.
For experiment A, we have:
[A⁻]/[HA] = [CH₃COO⁻]/[CH₃COOH] = (0.1 M)/(0.1 M) = 1
pH = pKa + log([A⁻]/[HA]) = 4.75 + log(1) = 4.75
The expected pH value for experiment A is 4.75.
For experiment B, we have:
[A⁻]/[HA] = [CH₃COO⁻]/[CH₃COOH] = (0.1 M)/(1.0 M) = 0.1
pH = pKa + log([A⁻]/[HA]) = 4.75 + log(0.1) = 4.25
The expected pH value for experiment B is 4.25.
For experiment C, we have:
[A⁻]/[HA] = [HCO₃⁻]/[H₂CO₃] = (0.1 M)/(0.1 M) = 1
pH = pKa + log([A⁻]/[HA]) = 6.37 + log(1) = 6.37
The expected pH value for experiment C is 6.37.
For experiment D, we have:
[A⁻]/[HA] = [HCO₃⁻]/[H₂CO₃] = (0.1 M)/(1.0 M) = 0.1
pH = pKa + log([A⁻]/[HA]) = 6.37 + log(0.1) = 5.77
The expected pH value for experiment D is 5.77.
In summary, the expected pH values of the buffer systems from the experiments are:
A: 4.75
B: 4.25
C: 6.37
D: 5.77
To know more about pH, refer
https://brainly.com/question/172153
#SPJ11
Why does sugar dissolve
Answers
Answer:
Sugar dissolves because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules.
Explanation:
An advantage of using renewable energies is? 1.They are harmful to the environment 2.We have a limited supply of them 3.They allow us to take energy from natural processes
Answers
Answer:
3. They allow us to take energy from natural processes
Explanation:
There are two major sources of energy namely: renewable and non-renewable. Renewable sources of energy are those natural sources that gets replenished faster than they are utilized, which is contrary to non-renewable sources. Examples of renewable sources of energy are wind energy, solar energy, hydro (water) energy.
According to the question, one advantage or merit that renewable sources have over their counterparts is that they allow us to take energy from natural processes e.g. wind, sunlight etc.
Which describes the molecule shown below?
O A. Unsaturated alkyne
O B. Saturated alkane
O C. Saturated alkene
O D. Unsaturated alkane

Answers
The description of the molecules shown is it is unsaturated alkynes. The correct option is A.
What are unsaturated alkynes?Alkynes are unsaturated double bond containing compound that react with hydrogen when a catalyst is present.
The pie bond shows that the hydrogen atoms are lost from the alkanes.
Thus, the correct option is A, unsaturated alkynes.
Learn more about unsaturated alkynes
https://brainly.com/question/23508203
#SPJ2
Consider the measurements shown. The
most precise value is highlighted for
each measurement.
9.3478 m + 2.73 m =
What place value must the answer be
reported to?
1. ones place
2. tenths place
3. hundredths place 4. thousandths place

Answers
The solution must be stated to the tenths place as follows: 9.3478 + 2.73 = 12.08.
In decimal numbers, what does place mean?After the decimal, the tenths place is represented by the first digit. After the decimal, the hundredths place is represented by the following digit. The place values are filled in by the remaining digits until there are none left.
As stated below, 2.73 must be added by 9.3478.
9.3478 has five significant figures, compared to 2.73's three.
Addition results in 12.0778.
The output will have decimal places equal to the lowest one of the given numbers, according to the criteria for adding significant figures.
Therefore, there should be two decimal places in this result.
We will now wrap things up. The response will be 12.08 because there is a 7 in third position.
9.3478 + 2.73 = 12.08
To know more about decimal place value visit :-
https://brainly.com/question/20563248
#SPJ13
When alkaline hydrolysis was first invented what jobs were people hiring to do?
Answers
When alkaline hydrolysis was first invented, people were hired for various roles related to the process and implementation of this technology. Some of the jobs that emerged include Chemical engineers, Technicians and operators, Waste management specialists, Scientists and researchers.
Chemical engineers: These professionals played a crucial role in developing and optimizing the alkaline hydrolysis process. They were responsible for designing the equipment, developing the necessary chemical reactions, and ensuring the efficient operation of the system.
Technicians and operators: Skilled technicians and operators were hired to operate and maintain the alkaline hydrolysis equipment. They were trained to monitor the process parameters, handle the chemicals involved, and ensure the proper functioning of the system.
Waste management specialists: With the introduction of alkaline hydrolysis as a method for disposal of organic waste, specialized professionals in waste management were employed to oversee the proper handling and treatment of the waste materials. They were responsible for implementing safety protocols, managing waste streams, and complying with environmental regulations.
Scientists and researchers: Alkaline hydrolysis required scientific expertise for continuous improvement and innovation. Scientists and researchers were hired to study the process, analyze the results, and explore potential applications in various fields such as biofuel production and chemical synthesis.
Overall, the introduction of alkaline hydrolysis created employment opportunities for professionals in engineering, chemistry, waste management, and research, among others, as this technology gained recognition and adoption.
To know more about hydrolysis , click here, https://brainly.com/question/31132313
#SPJ11
What is the Ka of a 0.0796 M solution of nitrous acid (HNO2) with a pH of 2.95?

Answers
Answer:
Coefficient = 1.58
Exponent = - 5
Explanation:
pH = 2.95
Molar concentration = 0.0796M
Ka = [H+]^2 / [HA]
Ka = [H+]^2 / 0.0796
Therefore ;
[H+] = 10^-2.95
[H+] = 0.0011220 = 1.122 × 10^-3
Ka = [H+] / molar concentration
Ka = [1.122 × 10^-3]^2 / 0.0796
Ka = (1.258884 × 10^-6) / 0.0796
Ka = 15.815 × 10^-6
Ka = 1.58 × 10^-5
Coefficient = 1.58
Exponent = - 5
Calculate the molarity of a solution made by dissolving 11.8 g of NaOH (solid) in sufficient water .199 M NaOH .196 M NaOH .198 M NaOH .197 M NaOH
Answers
0.197 M NaOH is the molarity of a solution made by dissolving 11.8 g of NaOH (solid) in sufficient water.
Thus, The total number of moles of solute in a given solution's molarity is expressed as moles of solute per liter of solution.
As opposed to mass, which fluctuates with changes in the system's physical circumstances, the volume of a solution depends on changes in the system's physical conditions, such as pressure and temperature.
M, sometimes known as a molar, stands for molarity.
When one gram of solute dissolves in one litre of solution, the solution has a molarity of one. Since the solvent and solute combine to form a solution in a solution, the total volume of the solution is measured.
Thus, 0.197 M NaOH is the molarity of a solution made by dissolving 11.8 g of NaOH (solid) in sufficient water.
Learn more about Molarity, refer to the link:
https://brainly.com/question/31545539
#SPJ1
what is phase change?
Answers
Explanation:
In chemistry, thermodynamics, and many other related fields, phase transitions (or phase changes) are the physical processes of transition between the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
Answer:
In chemistry, thermodynamics, and many other related fields, phase transitions are the physical processes of transition between the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases. A phase of a thermodynamic system and the states of matter have uniform physical properties.
Explanation:
Just need the bottom two rows completed. Will give brainliest.

Answers
Explanation:
19/9 F -1 (19 is stacked over 9 next to F) #protons: 9, #neutrons: 10, #electrons: 10
55/26 Fe +3, atomic #: 26, mass #: 55, #protons: 26, #neutrons: 29, #electrons: 23
How do we know what stars are made of?
1. Add spectroscopy evidence and describe the technology of seeing spectral lines even that are NOT in the visible range of our eyes.
2. What hot gasses is our Sun made of? How do we know? Include the spectral lines from the sun and the individual gasses for a match
3. Choose another space body: a star or nebula in deep space, and identify its composition and what technology was used to view it.
Answers
These observational techniques and technologies enable astronomers to unravel the complex composition of stars and celestial objects, shedding light on the mysteries of the universe.
1. We know what stars are made of through the use of spectroscopy. Spectroscopy is a scientific technique that analyzes the interaction between light and matter. It allows us to study the unique fingerprint of light emitted or absorbed by different elements.
By using spectroscopy, scientists can examine the spectral lines, which are specific wavelengths of light that are either emitted or absorbed by different elements. These spectral lines provide crucial information about the chemical composition of stars and other celestial objects.
Spectroscopy extends beyond the visible range of our eyes. There are different types of spectroscopy, such as ultraviolet, infrared, and X-ray spectroscopy, which allow us to observe spectral lines that are not visible to us directly. These technologies use specialized detectors and instruments to detect and analyze these wavelengths of light, providing valuable insights into the composition of stars and other objects.
2. Our Sun is primarily composed of hot gases. Through spectroscopy, scientists have identified the specific elements present in the Sun's atmosphere. The prominent spectral lines observed in the Sun's spectrum correspond to elements such as hydrogen, helium, and trace amounts of other elements like oxygen, carbon, and iron.
The spectral lines from the Sun match with known spectral lines of these elements, confirming their presence in the Sun's composition. By studying the intensity and characteristics of these spectral lines, scientists can deduce the abundance and temperature of the different gases in the Sun.
3. Let's consider the Orion Nebula as an example of a deep space object. The composition of the Orion Nebula has been studied using a combination of technologies, including optical spectroscopy and infrared observations.
Optical spectroscopy helps to identify the presence of elements such as hydrogen, helium, oxygen, nitrogen, and other trace elements in the nebula. By analyzing the spectral lines emitted or absorbed by these elements, scientists can determine their abundance and temperature.
Infrared observations, on the other hand, allow scientists to probe the dust particles present in the nebula. By studying the infrared emission from the dust, scientists can gain insights into the chemical composition of the interstellar material and molecules present in the Orion Nebula.
Learn more about celestial objects here :-
https://brainly.com/question/16629339
#SPJ11
Calculate the number of atoms of each element present in each of the following samples. a. 4.21 g of water b. 6.81 g of carbon dioxide c. 0.000221 g of benzene, C6H6 d. 2.26 moles of C12H22011
Answers
The number of atoms of each element present are 1.4 × 10²³ atoms of water, 9.27 × 10²² atoms of carbon dioxide, 1.62 × 10¹⁸ atoms of benzene and 1.36 × 10²⁴ atoms of sucrose.
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter.
A mole is defined as the amount of substance containing the same number of atoms, molecules, ions, etc. as the number of atoms in a sample of pure 12C weighing exactly 12 g.
a. Mass of water = 4.21g
Moles = 4.21 / 18
= 0.233 moles
number of atoms = 6.023 × 10²³ × 0.233
= 1.4 × 10²³ atoms.
b. mass of carbon dioxide = 6.81g
moles = 6.81 / 44
= 0.154 moles
number of atoms = 6.023 × 10²³ × 0.154
= 9.27 × 10²² atoms.
c. mass of benzene = 0.00021g
moles = 0.00021 / 78
= 2.69 × 10⁻⁶ moles
number of atoms = 6.023 × 10²³ × 2.69 × 10⁻⁶
= 1.62 × 10¹⁸ atoms.
d. 2.26 moles
number of atoms = 6.023 × 10²³ × 2.26
= 1.36 × 10²⁴ atoms.
Learn more about Moles, here:
https://brainly.com/question/31597231
#SPJ1
how many moles are needed to make 2.5 L of a 3.8 M solution?
Answers
Answer:
9.5
Explanation:
that the answer I hope am correct
Which compound undergoes solvolysis in aqueous ethanol most rapidly and why? Remember: solvolysis refers to ionization of the molecule aided by the solvent. a. cyclohexyl bromide b. isopropyl chloride c. methyl iodide d. 3-chloropentane e. 3-iodo-3-methylpentane
Answers
In solvolysis, the carbon-halogen (C-X) bond is broken in presence of water to form a carbon-oxygen (C-OH) bond.
From the given options, 3-iodo-3-methylpentane undergoes solvolysis in aqueous ethanol most rapidly because;
1. Iodine has a larger atomic radius compared to bromine and chlorine and this forms weaker carbon-halogen (C----I) bonds, which are easier to break during solvolysis.
2. 3-iodo-3-methylpentane has a tertiary carbon atom (R3-C---I) bonded to the iodine. Tertiary carbocations (R3-C+) are more stable due to hyperconjugation and inductive effects, which allows the reaction to proceed faster compared to primary and secondary carbocations.
Therefore, the weak C---I bond and the stability of the tertiary carbocation (R3-C+) formed, contribute to the rapid solvolysis of 3-iodo-3-methylpentane in aqueous ethanol.
https://brainly.com/question/31485910
#SPJ11
Why does an iron is called an element?
Answers
the energy of activation (Ea) for a certain biological reaction is 50 kJ/mol, by what factor will the rate of this reaction increase when body temperature increases from 37 degrees Celsius (normal) to 40 degrees Celsius (fever)?
Answers
The rate of the biological reaction will increase by a factor of 0.047 (or about 4.7%) when the body temperature increases from 37 degrees Celsius to 40 degrees Celsius.
The energy of activation (Ea) is the amount of energy that needs to be supplied to a chemical reaction in order for it to occur. In other words, it's the energy barrier that must be overcome for reactants to transform into products. When the temperature is increased, the energy of the reactant molecules also increases. The probability of a successful collision between the reactant molecules increases, which means that the reaction rate will also increase.
In this case, the energy of activation for the biological reaction is 50 kJ/mol. The rate of this reaction can be expressed using the Arrhenius equation:
k = A * e^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor (a constant that depends on the specific reaction), R is the gas constant, and T is the absolute temperature.
Assuming that the pre-exponential factor and the gas constant remain constant, we can calculate the ratio of the rate constant at 40 degrees Celsius (313 K) to the rate constant at 37 degrees Celsius (310 K) as follows:
k2/k1 = e^(-Ea/R * (1/T2 - 1/T1))
k2/k1 = e^(-50000/8.314 * (1/313 - 1/310))
k2/k1 = e^(-3.057)
k2/k1 = 0.047
The rate of the reaction is dependent on the energy of activation, which can be overcome by increasing the temperature of the system. A slight increase in temperature, such as during a fever, can increase the rate of the reaction by a small factor.
For such more questions on reaction
https://brainly.com/question/29470602
#SPJ11
What’s the answer??????
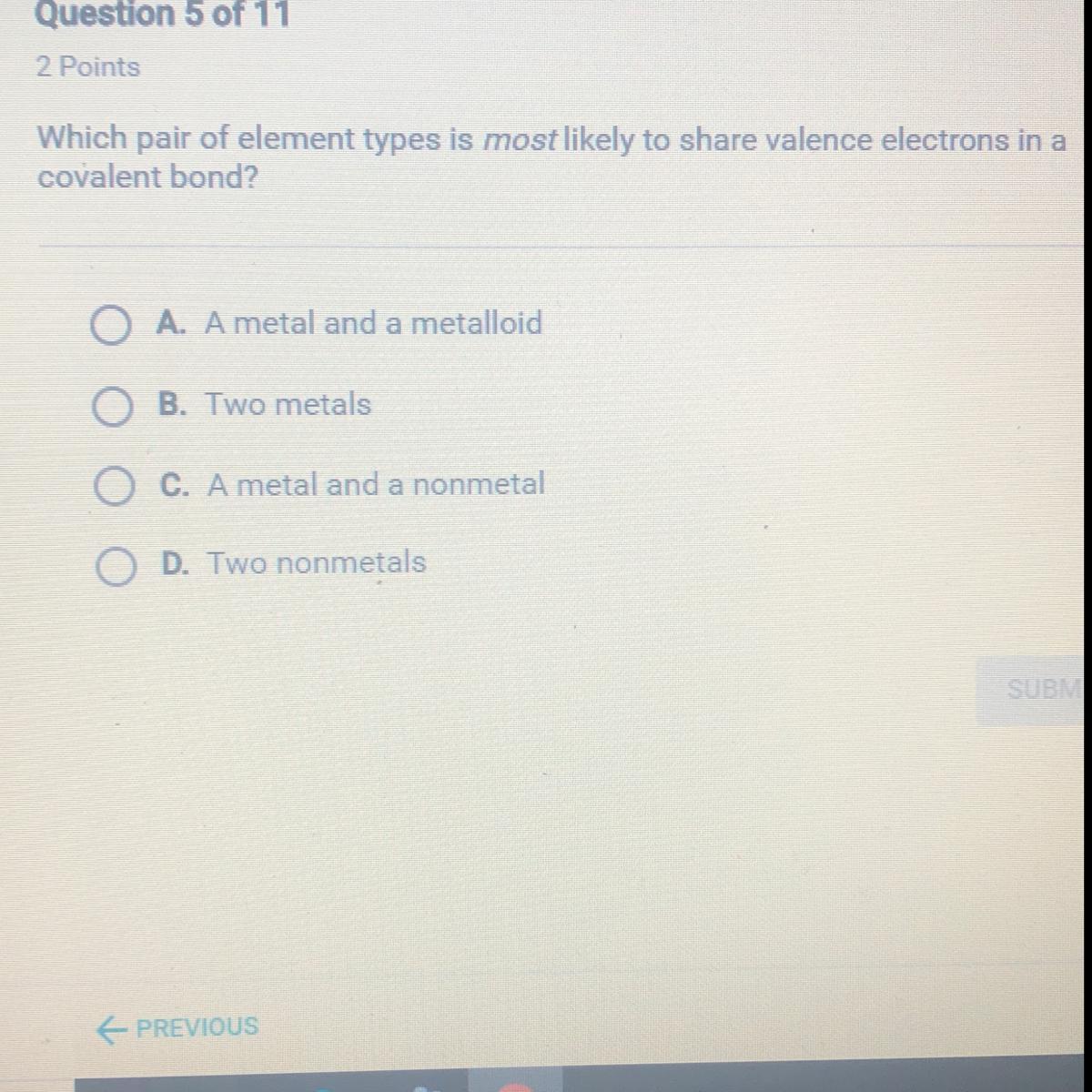
Answers
Answer:
D. Two non-metals
Explanation:
Non metals mostly form covalent bonds where they share electrons whereas, metal and nonmetal form ionic bonds where electrons are transferred.
Name the two types of sub-atomic particle in the nucleus of an atom.
Answers
Hope it helps you!!!!
What value must you identify before measuring the absorbance of your diluted solutions?
a.????max
b.molar absorptivity
c.path length
d.concentration of spinach extract solution
Answers
Before measuring the absorbance of a diluted solution using a spectrophotometer, it is necessary to identify the path length of the sample cell. The path length refers to the distance that light travels through the solution in the cell, and it is typically measured in centimeters.
The absorbance of a solution depends on the concentration of the absorbing substance, the path length of the cell, and the molar absorptivity of the substance at a given wavelength. The molar absorptivity, also known as the extinction coefficient, is a constant that reflects the efficiency of a substance in absorbing light at a specific wavelength.
However, in order to use Beer's Law (A = εbc) to calculate the concentration of the absorbing substance in a solution, it is necessary to know the path length (b) of the cell. Therefore, it is important to measure or know the path length of the cell before measuring the absorbance of the diluted solutions in order to calculate the concentration of the absorbing substance accurately.
To learn more about diluted solutions refer to:
brainly.com/question/1416865
#SPJ4
in the lab, john has two solutions that contain alcohol and is mixing them with each other. he uses milliliters less of solution a than solution b. solution a is alcohol and solution b is alcohol. how many milliliters of solution b does he use, if the resulting mixture has milliliters of pure alcohol?
Answers
To determine the number of milliliters of solution B used by John, we need to consider that the resulting mixture has a certain volume of pure alcohol. Additionally, it is mentioned that solution A contains alcohol and that John uses fewer milliliters of solution A than solution B. By comparing the volumes of pure alcohol in each solution and considering their respective concentrations, we can calculate the amount of solution B used by John.
Let's assume that John uses x milliliters of solution B. Since he uses fewer milliliters of solution A, the volume of solution A used would be x - y, where y represents the milliliters less of solution A used.
To determine the amount of pure alcohol in each solution, we need to consider their concentrations. Let's denote the concentration of solution A as A and the concentration of solution B as B. Given that the resulting mixture contains z milliliters of pure alcohol, we can set up the equation:
(A * (x - y)) + (B * x) = z
Simplifying the equation, we can solve for x:
Ax - Ay + Bx = z
(A + B) * x - Ay = z
x = (z + Ay) / (A + B)
By substituting the given values of y, A, B, and z into the equation, we can determine the number of milliliters of solution B used by John.
To know more about Pure alcohol :
brainly.com/question/14113912
#SPJ11
what is the mass of 3.8 mol of the element iron(Fe )?
