Answers
Disaccharides are sugars made up of two saccharides units linked together by a COC bond.
What is the purpose of a disaccharide?Like any other carbohydrate, disaccharides serve as the body's energy source. Disaccharides are converted by our bodies into simple sugars (simple sugars) for the small intestine to absorb when we ingest meals containing them.
What does it mean to be a disaccharide?Disaccharide, commonly known as double sugar, is any material made up of two simple sugar molecules (monosaccharides) joined together.
To know more about disaccharides visit:
https://brainly.com/question/16580858
#SPJ1
Related Questions
What is the formula for calculating pH?
O A. pOH= [H*]
————
[OH -]
B. pH = -log[OH-]
C. pH = -log[H+]
D. pH = log[ht]
Answers
Answer:
c. pH=-log[H+]
Explanation:
pH=-log10[H+]
Venus's atmosphere, while primarily CO2, is also 3.5% nitrogen gas (i.e. mole fraction of 0.035). What is the partial pressure of nitrogen on Venus in kPa given that the total atmospheric pressure is 1334 psi?
Answers
The partial pressure of nitrogen on Venus is approximately 321.914 kPa.
To find the partial pressure of nitrogen on Venus, we need to calculate the partial pressure using the mole fraction of nitrogen and the total atmospheric pressure. First, we convert the total atmospheric pressure from psi to kilopascals (kPa) since the mole fraction is given in terms of kPa.
1 psi = 6.89476 kPa
Therefore, the total atmospheric pressure on Venus is:
1334 psi × 6.89476 kPa/psi = 9197.53 kPa
Next, we can calculate the partial pressure of nitrogen using the mole fraction. The mole fraction of nitrogen is given as 0.035, which means that nitrogen makes up 3.5% of the total moles of gas in the atmosphere.
The partial pressure of nitrogen is given by:
Partial pressure of nitrogen = Mole fraction of nitrogen × Total atmospheric pressure
Partial pressure of nitrogen = 0.035 × 9197.53 kPa
Partial pressure of nitrogen = 321.914 kPa
Therefore, the partial pressure of nitrogen on Venus is approximately 321.914 kPa.
It's important to note that the given atmospheric composition of Venus's atmosphere and the total atmospheric pressure are approximate values and can vary depending on specific conditions and measurements.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
Every neutral atom of a given element has the same number of what two subatomic particles?
A. Protons and neutrons
B. Protons and electrons
Answers
Answer:
protons and electrons
Explanation:
because two atoms of the same elements have different(mass)isotope so the answer is B
at a blood bank there are five problems with a labortory technician's work performance the first year of work
Answers
The performance of a laboratory technician in a blood bank is crucial as it directly impacts the quality of the blood products and patient safety. If there are five problems with a technician's work performance in the first year of work, it can have serious consequences for the blood bank's operations.
Some of the potential problems that may arise include:
Improper labeling of blood products: This can result in confusion and incorrect transfusions.
Mishandling of blood products: This can lead to contamination, spoilage, or improper storage, which can affect the quality of the blood products.
Failure to follow standard operating procedures: This can result in errors, deviations from protocols, and potential safety hazards.
Poor communication skills: This can result in misunderstandings, delays, and errors in documentation.
Inadequate training or knowledge: This can lead to mistakes, misinterpretation of test results, and failure to recognize potential problems.
for more such questions on laboratory
https://brainly.com/question/29482908
#SPJ11
CAN SOMEONE HELP WITH THIS QUESTION BELOW?✨
If a plot of weight (in g) vs. volume (in mL) for a metal gave the equation y=15.28x and R^2=0.9981, what is the density of the metal?
Answers
Answer:- density = 13.41 g/ml
Density (d) of a substance is the mass (m) occupied by it in a given volume (v).
Density mass/volume =
i.e. d = m/v
m = (d) v -----(1)
The given equation from the plot of weight vs volume is :
y = 13.41 x ---- (2)
Based on equations (1) and (2) we can deduce that the density of the metal is 13.41 g/ml
Americium-241 is widely used in smoke detectors. The radiation released by this element ionizes particles that are then detected by a charged-particle collector. The half-life of 241Am is 432 years, and it decays by emitting alpha particles. How many alpha particles are emitted each second by a 5.54-g sample of 241Am
Answers
Answer:
Explanation:
Half life T is 432 years .
432 years = 432 x 365 x 24 x 60 x 60 s
= 1.36 x 10¹⁰ s .
No of atoms in 5.54 g of sample of Am N= 6.02 x 10²³ x 5.54 / 241
N = 138.38 x 10²⁰ atoms .
disintegration constant λ = .693 / half life
λ = .693 / 1.36 x 10¹⁰ s .
= .51 x 10⁻¹⁰ .
dN / dt = λN
= .51 x 10⁻¹⁰ x 138.38 x 10²⁰
= 70.57 x 10¹⁰ per second.
No of particles emitted = no of disintegration per second
No of particles emitted = 70.57 x 10¹⁰ per second.
Two elevators carry five passengers to the fifth floor. However, the elevators do not do the same work. Choose the best factor for
determining the amount of work the elevators did.
A.the speed of the elevator
B.the weight of the passengers
C.the number of buttons pressed
Will mark brainlist pls help!
Answers
Answer:
B the weight of the passengers
based on the half-life of Tc-99, how many half-lives have to pass for a 150 mg sample of Tc-99 to decay down to 30mg?
Answers
Answer:
2.322 half-lives have passed to decay down from 150 miligrams to 30 miligrams.
Explanation:
The half of Technetium-99 is approximately 211000 years. The decay of isotopes is represented by the following ordinary differential equation:
\(\frac{dm}{dt} = -\frac{m}{\tau}\) (Eq. 1)
Where:
\(\frac{dm}{dt}\) - First derivative of isotope mass in time, measured in miligrams per year.
\(m\) - Mass of the isotope, measured in miligrams.
\(\tau\) - Time constant, measured in years.
Now we proceed to obtain the solution of this differential equation:
\(\frac{dm}{m} = -\frac{dt}{\tau}\)
\(\int {\frac{dm}{m} } = -\frac{1}{\tau}\int dt\)
\(\ln m = -\frac{t}{\tau}+C\)
\(m(t) = m_{o}\cdot e^{-\frac{t}{\tau} }\) (Eq. 2)
Where:
\(m_{o}\) - Initial mass of the isotope, measured in miligrams.
\(t\) - Time, measured in years.
The time passed for isotope is cleared within the equation described above:
\(\ln \frac{m}{m_{o}} = -\frac{t}{\tau}\)
\(t = -\tau \cdot \ln \frac{m}{m_{o}}\)
In addition, we can obtain the time constant as a function of half-life:
\(\tau = \frac{t_{1/2}}{\ln 2}\) (Eq. 3)
If we know that \(t_{1/2} = 211000\,yr\), \(m_{o} = 150\,mg\) and \(m = 30\,mg\), then the time passed is:
\(\tau = \frac{211000\,yr}{\ln 2}\)
\(\tau \approx 304408.654\,yr\)
\(t = -(304408.654\,yr)\cdot \ln \left(\frac{30\,mg}{150\,mg} \right)\)
\(t \approx 489926.829\,yr\)
The amount of passed half-lives is that time divided by a half-life. That is:
\(n = \frac{489926.829\,yr}{211000\,yr}\)
\(n = 2.322\)
2.322 half-lives have passed to decay down from 150 miligrams to 30 miligrams.
An iron ore sample contains Fe2O3
together with other substances. Reaction of the ore with CO
produces iron metal: αFe2O3(s)+βCO(g)→γFe(s)+δCO2(g)
Part A
Part complete
Balance this equation.
Express your answer as a balanced chemical equation. Identify all of the phases in your answer.
Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g)
Previous Answers
Correct
Part B
Calculate the number of grams of CO
that can react with 0.400 kg
of Fe2O3
.
Activate to select the appropriates template from the following choices. Operate up and down arrow for selection and press enter to choose the input value typeActivate to select the appropriates symbol from the following choices. Operate up and down arrow for selection and press enter to choose the input value type
m
=
nothing
g
Request Answer
Part C
Calculate the number of grams of Fe
formed when 0.400 kg
of Fe2O3
reacts.
Activate to select the appropriates template from the following choices. Operate up and down arrow for selection and press enter to choose the input value typeActivate to select the appropriates symbol from the following choices. Operate up and down arrow for selection and press enter to choose the input value type
m
=
nothing
g
Request Answer
Part D
Calculate the number of grams of CO2
formed when 0.400 kg
of Fe2O3
reacts.
Activate to select the appropriates template from the following choices. Operate up and down arrow for selection and press enter to choose the input value typeActivate to select the appropriates symbol from the following choices. Operate up and down arrow for selection and press enter to choose the input value type
m
=
nothing
g

Answers
From the question;
Part A
The balanced reaction equation is Fe2O3 + 3CO → 2Fe + 3CO2
Part B
The mass of the CO is 210 g
Part C
The mass of iron is 280 g
Part D
The mass of the carbon dioxide is 330 g
What is the stoichiometry?
The reaction equation is;
Fe2O3 + 3CO → 2Fe + 3CO2
Number of moles of the iron III oxide = 400 g/160 g/mol
= 2.5 moles
If 1 mole of iron III oxide reacts with 3 moles of carbon monoxide
2.5 moles of iron III oxide reacts with 2.5 * 3/1
= 7.5 moles
Mass of carbon monoxide = 7.5 moles * 28 g/mol
= 210 g
If 1 mole of iron III oxide form 2 moles of Fe
2.5 molees of iron III oxide will form
2.5 * 2/1
= 5 moles
Mass of iron = 5 moles * 56 g/mol
= 280 g
If 1 mole of iron III oxide forms 3 moles of carbon dioxide
2.5 moles of iron oxide will form 2.5 * 3/1
= 7.5 moles
Mass of the carbon dioxide = 7.5 moles * 44 g/mol
= 330 g
Learn more about stoichiometry:https://brainly.com/question/28780091
#SPJ1
what are 2 ways that all hydrocarbons are alike?
Answers
Answer:
Composition: All hydrocarbons are made up of only two types of atoms: carbon and hydrogen. They are like building blocks that contain carbon and hydrogen stuck together.
Organic Nature: Hydrocarbons are special because they are part of a group of compounds that come from living things or things that were once alive. They have carbon and hydrogen in them, which is what makes them different from other types of compounds.
Explanation:
Help Please!
Imagine a deer that lives in the meadow. What does the deer eat?
What eats the deer?
Answers
Answer: A deer that lives in a meadow would likely eat a variety of plants, such as grasses, leaves, and shrubs. Depending on the season and the availability of food, the deer's diet may also include fruits, nuts, and other plant materials. In some cases, deer may also eat insects or other small animals if they are available.
As for what eats the deer, there are many potential predators depending on the location and environment of the meadow. In some areas, predators of deer could include wolves, coyotes, cougars, or bears. In other areas, deer may be hunted by humans. In addition, some birds of prey, such as eagles or hawks, may also prey on deer, particularly fawns or other young deer.
Explanation:
If a less concentrated initial solution of sodium bicarbonate was used in beaker C, would that solution require more or less bicarbonate to neutralize the acid? Why?
Answers
If a less concentrated initial solution of sodium bicarbonate was used in beaker C, it would require more bicarbonate to neutralize the acid.
What is concentrated?Concentrated means something that has been increased in strength or power by reducing its volume. It can refer to a solution that has a higher concentration of solutes than the original solution, a sound that is louder or stronger, or a force that is more powerful or intense. Concentrated can also refer to a person’s focus or attention on one particular thing, when their thoughts and energy are directed to a single point.
This is because the concentration of sodium bicarbonate determines how much of the acid can be neutralized by the solution. If the initial solution is less concentrated, then it will take more of the bicarbonate to neutralize the same amount of acid.
To learn more about concentrated
https://brainly.com/question/30558048
#SPJ1
Cyclohexanecarboxylic acid, C6H11COOH (pKa 4.90), is only slightly soluble in water, but its sodium salt, C6H11COO-Na , is quite soluble in water. Describe the solubility of cyclohexanecarboxylic acid in solutions of sodium hydroxide, sodium bicarbonate (NaHCO3), and sodium carbonate (Na2CO3). The pKa values for the conjugate acids of sodium hydroxide, sodium bicarbonate (NaHCO3), and sodium carbonate (Na2CO3) are 15.7, 6.36, and 10.33, respectively.
Answers
Answer:
See explanation
Explanation:
Carboxylic acids are all soluble in solutions of NaOH, Na2CO3 and NaHCO3 due to the formation of a sodium salt of the acid.
In all these cases, the sodium salt of cyclohexanecarboxylicacid formed is more soluble in water than the parent acid.
When the acid is dissolved in sodium bicarbonate or sodium carbonate, carbon dioxide gas is also evolved.
Which type of stars have the highest luminosity?
A. main sequence
B. blue giants
C. red super giants
D. BOTH blue giants and red super giants
Answers
Answer: Red supergiant.
Explanation:
The stars in group C are even more luminous than the giants. These are super giants, the largest of stars with extremely high luminosisties. A red supergiant such as Betelguese would extend beyond the orbit.
a graduated cylinder has a mass of 50 g when empty. When 30 mL of water is added the graduated cylinder has a mass of 120 g. If a rock is added to the graduated cylinder, the water level rises to 75 mL and the total mass is now 250 g. What is the dentisy of the rock?
Answers
The density of the solid object is obtained to be 2.89 g/mL.
What is density?The density is defined as the ratio of the mass to the volume of the object. In this case, the density is to be obtained by the use of the displacement method of the solving the problem.
Mass of the empty cylinder = 50 g
Mass of water and cylinder = 120 g
Mass of water alone = 70 g
Mass of water, cylinder and the object = 250 g
Mass of the object = 250 g - 120 g = 130 g
Volume of the object = 75 mL - 30 mL = 45 mL
Density of the object = 130 g/45 mL
= 2.89 g/mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
7.5 L of a gas at 2 ATM and a temperature of 75°C is changed and volume to 3.4 L and a pressure of .5 ATM what is the new temperature
Answers
Answer:
Explanation:
Combined Gas Law
T2= T1P2V2/ (P1V1) = 348.15 X .5 X 3.4/(2 X 7.5) =39.46 K or -233.69C
if two substance are at the same temperature, their enthalpy
Answers
Answer:
cannot be measure
Hope this helps :) !!!
Please help me :(
1.) A laser emits light of frequency = 4.74 x 1014 sec-1. What is the wavelength of the light in nm?
2.) A certain electromagnetic wave has a wavelength of 625 nm.
a.) What is the frequency of the wave?
b.) What region of the electromagnetic spectrum is it found?
c.) What is the energy of the wave?
Answers
If pollution near factories was reduced, what
would happen to the color of the moths
Answers
Answer:
It would most likely just change their color
Explanation: The frequency of dark-coloured moths increased at that time, an example of industrial melanism. Later, when pollution was reduced, the light-coloured form again predominated. ... The dark-coloured or melanic form of the peppered moth
If pollution near factories was reduced, then this situation increased the frequency of white moths.
What is industrial melanism?Industrial melanism is an evolutionary feature associated with the increase of dark moths in industrial polluted areas.
Industrial melanism is an adaptation to survive these industrial conditions.In conclusion, if pollution near factories was reduced, then this situation increased the frequency of white moths.
Learn more about industrial melanism here:
https://brainly.com/question/15283847
#SPJ2
estimate the number of atoms in your body. (hint: based on what you know about biology and chemistry, what are the most common types of atom in your body? what is the mass of each type of atom? appendix d gives the atomic masses of different elements, measured in atomic mass units; you can find the value of an atomic mass unit, or 1 u, in appendix e.)
Answers
It is difficult to estimate the exact number of atoms in a human body because number can vary greatly depending on a person's size and body composition. However, we can make an estimate based on the average mass of the human body and average atomic masses of the most common elements in the body.
The most common elements in the human body are oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. These elements make up approximately 99% of the total body mass.
Using the average atomic masses of these elements and assuming a body mass of 70 kg, we can estimate the number of atoms as follows:
Oxygen: approximately 25 kg, or 3.5 × 10⁻²⁶ atoms.
Carbon: approximately 18 kg, or 2.5 × 10⁻²⁶ atoms.
Hydrogen: approximately 10 kg, or 1.4 × 10⁻²⁶ atoms.
Nitrogen: approximately 3 kg, or 4.2 × 10⁻²⁵ atoms.
Calcium: approximately 1.5 kg, or 2.1 × 10⁻²⁵ atoms.
Phosphorus: approximately 0.8 kg, or 1.1 × 10⁻²⁵ atoms.
So, the total number of atoms in a 70 kg human body would be approximately 10⁻²⁷ to 10⁻²⁸ atoms.
The exact number of atoms in a human body can vary greatly depending on a number of factors, including age, body composition, and overall health.
To know more about atoms here
https://brainly.com/question/29695801
#SPJ4
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Consider the reaction A → products at 311 K. The concentration of A was monitored over time and the data was analyzed by plotting. It was found that a plot of 1/[A] vs time gave a straight line relationship. It was also observed that it took 24.5 s for the concentration of A to decrease from 0.757 M to 0.107 M. What is the half life for this reaction when [A]o = 0.757 M?
Answers
The half life for this reaction when [A]o = 0.757 M is 4.033 s
integrated rate law for 2nd order reaction:
1/[A]o = 1/[A] - k*t
so, for 2nd order reaction, 1/[A] vs t will be straight line
integrated rate law for 1st order reaction:
ln [A] = –kt + ln [A]o
So, for 1st order reaction, ln[A] vs t will be straight line
integrated rate law for zero order reaction:
[A] = [A]o – k*t
So, for zero order reaction, [A] vs t will be straight line
Here,
1/[A] vs t is straight line
so, order of A is 2
use integrated rate law for 2nd order reaction:
1/[A] = 1/[A]o + k*t
1/(0.107) = 1/(0.757) + k*24.5
9.346 = 1.321 +k*24.5
k*24.5 = 8.025
k = 0.328 M-1.s-1
Given:
rate constant, k = 0.328 M-1.s-1
use relation between rate constant and half life of 2nd order reaction
t1/2 = 1/([A]o*k)
= 1/(0.757*0.328)
= 4.033 s
The half-life of a chemical reaction can be defined as the time it takes for the concentration of a given reactant to reach 50% of its initial concentration (that is, the time it takes for the concentration of a reactant to reach half its initial value). This is represented by the symbol "t1/2" and is usually expressed in seconds.
Learn more about half life of a chemical reaction:
https://brainly.in/question/15482143
#SPJ4
Question 4 of 10
Which statement best describes how scientists and engineers work together
in the research and development cycle?
OA. Scientists develop a theory, and then engineers perform
experiments to verify its accuracy.
B. Engineers do experiments to answer questions about nature, and
then scientists use the results to create a new technology.
OC. Scientists make designs based on engineering knowledge and
then make it available for public use.
D. Engineers develop a new technology, and then scientists use it to
make a new discovery.
Answers
Answer: Scientists make designs based on engineering knowledge and
then make it available for public use.
Complete and balance the following chemical equations. Identify the reaction type as: combination, decomposition, single replacement, double replacement, or combustion.
Products:
Magnesium Oxide + Carbon dioxide.
a) MgCO₃ (Heat is supplied to the reaction (triangle over a arrow) -> Reaction type:
Products:
Aluminum Oxide
b) Al + O₂ -> Reaction type:
Answers
Answer:
the first one is a decomposition reaction
the second one is also a synthesis reaction
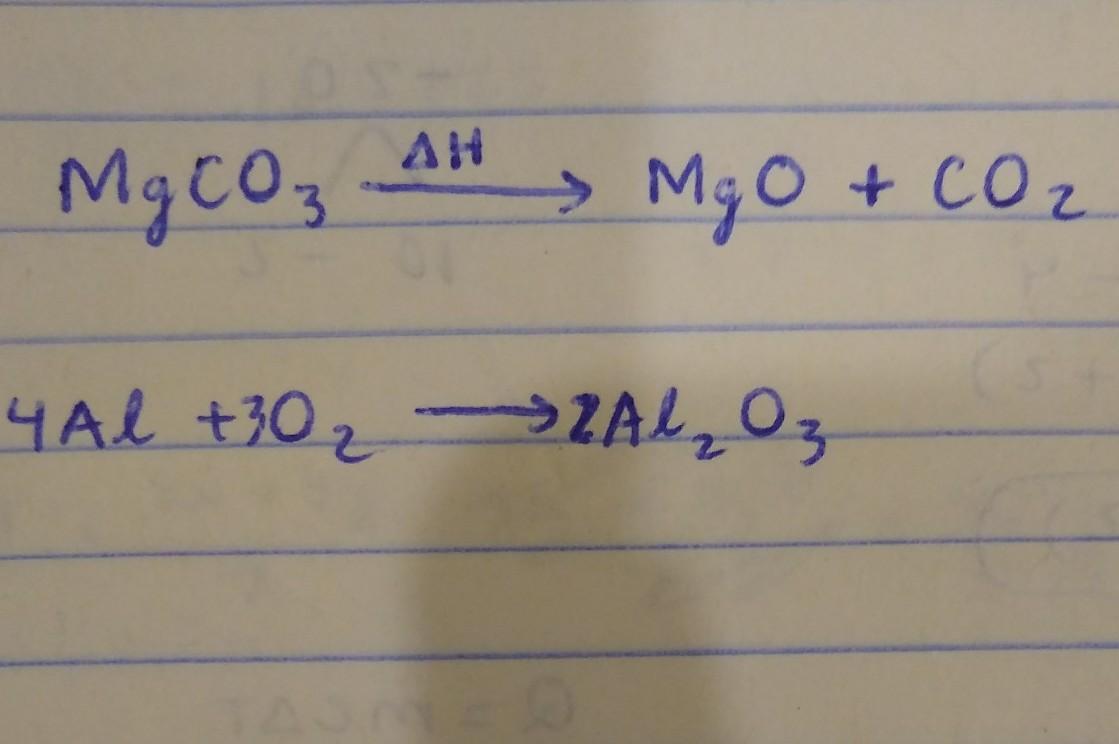
Solution:-1
\(\boxed{\sf {MgCO_3\atop Magnesium\:Carbonate}\overset{\Delta H}{\longrightarrow}{MgO\atop Magnesium \:Oxide}+{CO_2\atop Carbon\:Dioxide}}\)
It is a thermal decomposition reaction
Solution:-2:-
\(\boxed{\sf {4Al\atop Aluminium}+{3O_2\atop Oxygen}\longrightarrow{2Al_2O_3\atop Aluminium\:oxide}}\)
It is a combination reaction.
which of the followinh compounds has the highest percentage of hydrogen atoms by mass
Answers
Answer:
water
. Of the compounds listed in the question, water has the highest percent mass of hydrogen.
Hope it helps
As the climate warms, ice and
snow melt. This makes the
climate hotter, which then
melts more snow and ice. This
is an example of
A. positive feedback
B. negative feedback
C. neutrality
Answers
The scenario in which as the climate warms, ice and snow melt making the climate hotter, which then melts more snow and ice is an example of positive feedback.
The correct option is A.
What is positive feedback?Positive feedback refers to a process in which an initial change or disturbance in a system leads to an amplification or reinforcement of that change.
Considering the scenario of climate change, the example provided earlier is a demonstration of positive feedback.
As the climate warms, ice and snow melt, reducing the reflective surface and exposing darker surfaces like land or water. These darker surfaces absorb more sunlight, which leads to further warming and more melting of ice and snow. This cycle continues, causing a self-reinforcing effect that amplifies the initial warming.
Learn more about positive feedback at: https://brainly.com/question/28271726
#SPJ1
what is the chemical formula for the magnesium ion and the carbonate ion?
Answers
Answer: The formula for magnesium carbonate is MgCO_3.
Explanation:
A scientist found a piece of metal and wanted to identify it based on its density. The sample she found had a mass of 8g and a volume of 2ml. Can you identify the mystery metal the scientist found? (D=m/v)
HELP I NEED THIS WITHIN 10 MINUTES PLEASE!!!!!

Answers
Answer:
Iron with a density of 16g/cm3
Name the substance in red blood cells that carbon monoxide from cigarette smoke combines with.
Answers
The substance in red blood cells that carbon monoxide from cigarette smoke combines with is called hemoglobin.
Hemoglobin is a protein molecule found in red blood cells that is responsible for transporting oxygen from the lungs to the rest of the body. Hemoglobin consists of four subunits, each containing a heme group. The heme group contains an iron ion, which binds to oxygen molecules in the lungs.
Carbon monoxide (CO) from cigarette smoke can bind to the iron ion in the heme group with a much greater affinity than oxygen. When CO binds to hemoglobin, it forms a stable complex called carboxyhemoglobin (COHb).
This reduces the amount of hemoglobin available for binding with oxygen, which can lead to a reduction in the amount of oxygen that can be transported to the tissues.
The formation of COHb can also affect the release of oxygen from hemoglobin in the tissues. Hemoglobin releases oxygen in response to a decrease in oxygen concentration, which is sensed by the heme group. However, when CO binds to the heme group, it alters the shape of the hemoglobin molecule and reduces its ability to release oxygen.
As a result, the presence of carbon monoxide in the blood can lead to a range of health problems, including headaches, dizziness, shortness of breath, and even death in severe cases.
The best way to prevent carbon monoxide poisoning is to avoid exposure to smoke from tobacco products and other sources of combustion, such as gas heaters and stoves.
For more question on red blood cells click on
https://brainly.com/question/1407525
#SPJ11
Hyenas eat larger mammals like antelope and wildebeest, but will also eat lizards, birds, snakes, and insects. What type of teeth would you expect a hyena to have?
Answers
Answer:
Large Sharp Teeth
Explanation:
i got a 100 on my test!
Large Sharp Teeth !
i got it right on my quiz
