PLEASE IM VERY CONFUSED
Directions: Write the formulas of the reactants and products, including the symbols for the state,
(s), (l), (g), (aq) - then balance the equations.
8. When a solution of hydrogen chloride is added to solid sodium bicarbonate (NaHCO3), the products are carbon dioxide, water and aqueous sodium chloride.
9. Ethyl alcohol (a liquid), C2H6O, burns in air to produce carbon dioxide and gaseous water.
10. Solid titanium (IV) chloride reacts with water, forming solid titanium (IV) oxide and aqueous hydrogen chloride.
11. During photosynthesis in plants, carbon dioxide and water are converted into glucose, C6H12O6, and oxygen gas.
12. Solutions of calcium hydroxide, Ca(OH)2 and nitric acid, HNO3, react to produce water and aqueous calcium nitrate, Ca(NO3)2.
Answers
Answer:
8. the formula of the products are CO2(g) , H2O (l) and NaCl (aq)
Explanation:
s- solid
g- gas
l- liquid
aq- aqueous, means acid
These are the products of the equation, you can balance this by searching up a video on how to do so, or use a balance equations calculator online. Balancing is very easy tho.
Related Questions
How would you explain the path exhaled oxygen-poor and carbon dioxide-rich air takes to leave the body
Answers
The path of exhaled oxygen-poor and carbon dioxide-rich air leaving the body begins in the body's cells, where oxygen is used for cellular respiration, and carbon dioxide is produced as a waste product.
When we breathe in, our body takes in oxygen-rich air through the mouth or nose. This air then travels through the trachea, or windpipe, and into the lungs. In the lungs, the oxygen is transferred to the bloodstream while carbon dioxide is released from the blood and into the air sacs of the lungs.
Next, the oxygen-rich blood is pumped by the heart to different parts of the body, where it is used for energy production. As the body uses oxygen, it produces carbon dioxide as a waste product.
This carbon dioxide is carried by the bloodstream back to the lungs, where it is released into the air sacs. Finally, when we exhale, the air travels back up the trachea and out of the mouth or nose, carrying the carbon dioxide-rich and oxygen-poor air out of the body.
This process of inhaling oxygen and exhaling carbon dioxide is known as respiration and is essential for the proper functioning of the body's cells and organs.
Visit here to learn more about Respiration:
brainly.com/question/22673336
#SPJ11
Which statement about London dispersion forces is true?
Answers
The London dispersion force is "the weakest intermolecular force"
__ bonds are formed when __ atoms __ their electrons to ___ atoms. (its fill in the blank) the words i have to use to fill the spaces are Molecular Covalent Metal Non-Metal give up share pls help lol
Answers
Answer:
Covalent (molecular) bonds are formed when electrons are shared between non metal atoms
Explanation:
A chemical bond is the attraction between atoms, ions or molecules of different or the same element that enables the formation of chemical compounds.
Covalent (molecular) bonds are formed when electrons are shared between non metal atoms. Covalent bonds help the atoms to reach a stable state.
Example of covalent bonds are carbon monoxide (CO), iodine monobromide (IBr), H2 (hydrogen) and so on
How many moles of O2 are formed from 1.252 g of HgO?
Answers
Moles of Hg
Given mass/Molar mass1.252/200.590.006mol2 moles produce 1 mol O2
1 mol produces 1/2mol O_2
0.006 mol produces
0.006(0.5)0.003molWhat happens to initial reaction rate if enzyme concentration is tripled? (Assume conditions like those in the properties of enzymes lab, and assume that the pH and initial substrate concentration are constant.)A. The initial rate will increase by a factor of 9 because the rate is dependent on the square of the enzyme concentration.B. The initial rate will triple when enzyme concentration is tripled because the initial rate of reaction is linearly related to enzyme concentration.C. The fixed substrate concentration will hold the initial rate constant because only substrate concentration governs the reaction rate.D. The fixed pH will hold the initial rate constant because only pH governs the reaction rate.E. The initial rate will not change because the enzyme is saturated with substrate.
Answers
If enzyme concentration is tripled, the initial rate will triple when enzyme concentration is tripled because the initial rate of reaction is linearly related to enzyme concentration. The correct answer is option B.
The initial rate of reaction and the enzyme concentration are directly proportional to each other. When the enzyme concentration is tripled, the initial rate of reaction will triple as well.
Assuming that conditions are like those in the properties of enzymes lab and pH and initial substrate concentration are constant, the initial rate of reaction depends on the enzyme concentration. Enzyme concentration affects the rate of the reaction at the beginning of the reaction. When the enzyme concentration is increased, the number of active sites available for the reaction is also increased.
The rate of a reaction is affected by several factors such as temperature, pH, substrate concentration, and enzyme concentration. In most cases, an increase in the enzyme concentration leads to an increase in the rate of the reaction. However, there comes a time when the rate of reaction reaches a maximum point irrespective of the enzyme concentration. At this point, the enzyme is said to be saturated with substrate.
Learn more about enzyme concentration here: https://brainly.com/question/13445202
#SPJ11
Which of the following is the electrical charge on an atom that contains 6 electrons, 3 neutrons, and 4 protons?
Answers
Answer:
Its electrical charge is -2.
what, if any, conclusions can you draw about the phs of the different categories of common substances? a. ph of beverages
Answers
It is difficult to draw a conclusive statement about the pHs of different categories of common substances because the pH of a substance is dependent on its specific composition. However, it is possible to make generalizations about certain categories of substances.
The pH of beverages can vary widely depending on their composition. Some common beverages such as water, milk, and pure fruit juices tend to have a pH close to neutral (pH 7). Other beverages such as carbonated soft drinks, energy drinks, and sports drinks tend to be acidic (pH less than 7) due to the presence of acids such as citric acid, phosphoric acid, and carbonic acid. Alkaline water and some herbal teas may have a pH greater than 7.
For example, acidic beverages are substances often associated with a higher risk of dental erosion and may be more likely to cause stomach discomfort in some individuals. On the other hand, alkaline beverages may have potential health benefits, but further research is needed to fully understand their effects on the body.
Learn more about pm pH here.https://brainly.com/question/172153
#SPJ11
1. The motion of an object is described as 240 mph to the northeast. What form of
motion is being described?
a. Speed
b. Velocity
c. Acceleration
d. None of the above
Answers
The form of motion which is described is velocity as it is describing the speed as well as the direction.
What is motion?Motion is defined as a phenomenon which is described with respect to change in object's position with respect to time.It is given in terms of displacement,velocity,distance ,acceleration,speed.
Branch of physics which deals with motion of objects without any reference to it's cause is called kinematics while the branch which studies forces and their effects on motion is called as dynamics.
Motion applies to various systems like objects,bodies, matter particles ,radiation ,curvature.There are three laws of motion which are proposed by Newton.Classical mechanics is based on Newton's law of motion.Relativistic mechanics is based on modern kinematics.
Learn more about motion ,here:
https://brainly.com/question/5961485
#SPJ2
Write the balance equation for
CaCl2 + Na2C204 = CaC204 + NaCl
Answers
Answer:
CaCl2 + Na2C2O4=CaC2O4 + 2NaCl
a formula calls for 0.4 ml of a coloring solution. you have a 5 ml graduate calibrated from 1-5 ml in 1 ml units. assuming 5 is your multiple, how many ml of water, as your diluent, will you need to obtain the desired quantity of the coloring solution?
Answers
Let us add 2.4 mL of water in 0.4 ml of coloring solution
total volume of solution = 2.4 + 0.63 mL therefore,
0.4 mL of coloring solution now includes = 2.4 ml of water
or
1 mL of coloring solution includes = 4mL of water
measure 3 mL of coloring solution with water that is formed
therefore, this 3 mL will contain 0.4 mL of coloring solution
formula calls for 0.4 mL of a coloring solution
5-mL graduate calibrated from 2 to 10 mL in 1-mL units
Now,
We want to use an aliquot technique to measure 0.4 ml of coloring solution.
Because the calibrated graduate is used to measure 2ml to 10ml with 1ml as unit measure
let us add 2.4 mL of water in 0.4 ml of coloring solution
total volume of solution 2.4 + 0.63 mL
therefore,
0.4 mL of coloring solution now includes = 2.4ml of water
or
1 mL of coloring solution includes = 4 mL of water
Hence
measure 3 mL of coloring solution with water that is formed
Therefore, this 3 mL will contain 0.4 mL of coloring solution
Learn more about Concentration here:
https://brainly.com/question/17206790
#SPJ4
Elaborate the difference between NO and No.
Responses
A NO and No both symbolize the molecule nitrogen monoxide - the capitalization doesn't matter.NO and No both symbolize the molecule nitrogen monoxide - the capitalization doesn't matter.
B NO and No represent neither a compound, a molecule, nor an element. Both are not possible.NO and No represent neither a compound, a molecule, nor an element. Both are not possible.
C NO represents the molecule nitrogen monoxide while No represents the molecule sodium oxide.NO represents the molecule nitrogen monoxide while No represents the molecule sodium oxide.
D NO is the molecule nitrogen monoxide while No is the atomic symbol for the element nobelium.
Answers
Answer:
D
Explanation:
NO is the molecule nitrogen monoxide while No is the atomic symbol for the element nobelium
the mass of empty cylinder is 20 gram its mass when its filled completely with water is 30 gram and its mass when its filled completely with unknown liquid is 27 gram find the density of, the unknown liquid
Answers
Answer:
≈ 0.70 g/cm³ (answer rounded up to 2 decimal places)
Explanation:
The mass of empty cylinder is 20g
The mass of water filling the cylinder = 30g - 20g = 10g
The mass of unknown liquid filling the cylinder = 27g - 20g = 7g
Density of water = 997 kg/m³
Converting this density to g/cm³ we get;
1kg = 1000g , 1m³ = 1000000cm³
So density = \(\frac{997000}{1000000}\) g/cm³ = 0.997 g/cm³
So the volume of water occupied by 10g is;
10 × 0.997 = 9.97 cm³
This volume is also occupied by 7g of the unknown liquid.
Density = mass/volume
Density of the unknown liquid = 7g ÷ 9.97cm³ = 0.702106319 g/cm³ ≈ 0.70 g/cm³ (answer rounded up to 2 decimal places)
How much solute will remain undissolved when 180 g of potassium iodide is added in 100 cm3 of water at 30°C?
Answers
Answer:
\(m_{undissolved}=27g\)
Explanation:
Hello,
In this case, we first define the solubility as the maximum amount of a solute that is completely dissolved in an specific amount of solvent and it is temperature-dependent. Thus for potassium iodide, its solubility at 30°C is 153 g per 100 cm3 of water, therefore, with the given amount, the undissolved amount results:
\(m_{undissolved}=180g-153g=27g\)
Best regards.
What amount of a 70% acid solution must be mixed with a 20%
solution to produce 200 mL of a 45% solution?
Answers
Answer:
To determine the amount of a 70% acid solution and a 20% solution needed to produce a 45% solution, we can set up a system of equations based on the principles of concentration and volume.
Let's assume that x mL of the 70% acid solution needs to be mixed with (200 - x) mL of the 20% solution.
The total amount of acid in the resulting mixture can be calculated as follows:
0.70x + 0.20(200 - x) = 0.45(200)
Now, let's solve this equation to find the value of x:
0.70x + 40 - 0.20x = 90
0.70x - 0.20x = 90 - 40
0.50x = 50
x = 50 / 0.50
x = 100
Therefore,
100 mL of the 70% acid solution needs to be mixed with (200 - 100) = 100 mL of the 20% solution to produce 200 mL of a 45% solution
To know more about to find amounts of mixed solutions visit:
https://brainly.com/question/33531478
#SPJ11
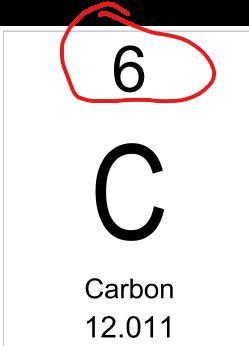
What number helps us identify the element?
Answers
Answer:
The Atomic Number
Explanation:
The atomic number is the number of protons of an atom in its nucleus.
Each element has a unique atomic number.
(view screenshot too, to find where it is in element table)
how to unclog a toilet without a plunger when the water is high
Answers
Answer: Use Hot Water.
Explanation:
To unclog a toilet without a plunger all u need to do is boil some water and carefully pour that into the toilet. Wait for some time and then pour some more hot water. Keep repeating this process till the water level starts going down.
Filter the air that we inhale and exhales carbon dioxide
Answers
Air filters are devices designed to remove impurities and particles from the air, improving its quality and making it healthier to breathe. While air filters can help remove certain contaminants, such as dust, pollen, and pet dander, they do not specifically filter out carbon dioxide (CO2).
Carbon dioxide is a natural component of the air we exhale, and its concentration in the atmosphere is regulated through natural processes, such as photosynthesis by plants. The removal of carbon dioxide from the air typically occurs through the natural carbon cycle, where plants absorb CO2 during photosynthesis and release oxygen.
If you are looking to reduce the carbon dioxide levels indoors, the most effective method is to ensure proper ventilation in the space. This can be achieved by opening windows or using mechanical ventilation systems to bring in fresh outdoor air and remove stale air. Additionally, increasing the number of plants indoors can help absorb carbon dioxide and release oxygen through photosynthesis.
It's important to note that while air filters can improve indoor air quality by removing various pollutants, they are not designed to specifically target or remove carbon dioxide.
Learn more about carbon dioxide Here-
https://brainly.com/question/14445045
#SPJ4
How is the Separation of a Compound different
from that of a mixture?
Answers
Suppoe that you ue 4. 25 g of Iron in the chemical reaction: 2Fe() 3Cu2(aq) rightward arrow 2Fe3(aq) 3Cu(). What i the theoretical yield of Cu(), in gram?
Answers
Theoretical yield of copper is 7.22 g when 4.25g of iron in the chemical reaction.
2Fe(s) + 3Cu2(aq.) ----> 2Fe3(aq.) + 3Cu (s)
Molar mass of Iron (Fe) is 56g/mole.
Molar mass of copper (cu) is 63.5g/mole.
2 mole ( 2 * 56g/mole) Iron produces = 3 mole ( 3 * 63.5g/mole ) copper
Theoretical yield is the maximum amount of product that could be formed from the given amounts of reactants. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. The theoretical yield is the amount of product that would be formed from a reaction if it was 100% efficient.
There are 4. 25 g of Iron .so,
4.25 g Iron produces = (3 * 63.5g / 2 * 56 g ) * 4.25 g copper
= 7.22 g copper
Theoretical yield of copper is 7.22g.
To learn more about Theoretical yield please visit:
https://brainly.com/question/2765357
#SPJ4
I am a alkaline earth metal.
A. Chromium
B. Molybdenum
C. Tungsten
D. Seaborgium
Answers
Answer:
C. Tungsten
Explanation:
Earth metals are magnesium and beryllium. The other three, which include rubidium, tungsten and cesium.
Please mark me as brainliest!
determine the poh in a 0.235 m naoh solution. a) 12 b) 0.63 c) 0.24 d) 13.3
Answers
The pOH of the solution is 0.63. the concentration of hydroxide ions in moles per liter.
To find the pOH in a 0.235 M NaOH solution, we need to use the equation :pOH = -log[OH-]where [OH-] represents the concentration of hydroxide ions in moles per liter (M).Step-by-step solution:To start, we need to determine the concentration of hydroxide ions [OH-] in the solution. The chemical formula for sodium hydroxide is NaOH. When dissolved in water, it dissociates into Na+ ions and OH- ions, as shown below: NaOH → Na+ + OH-This means that the concentration of hydroxide ions in the solution is the same as the concentration of sodium hydroxide, which is 0.235 M.So, [OH-] = 0.235 MNow we can use this value to calculate the pOH:pOH = -log[OH-]pOH = -log(0.235)pOH = 0.628. When rounded to two decimal places, the pOH of the solution is 0.63.So, the correct answer is option b) 0.63. We can write a 150 word answer as follows: A pH scale measures the concentration of hydrogen ions (H+) in a solution. The pOH is a measure of the concentration of hydroxide ions (OH-) in a solution. To calculate the pOH of a 0.235 M NaOH solution, we first need to determine the concentration of hydroxide ions. When sodium hydroxide (NaOH) is dissolved in water, it dissociates into Na+ ions and OH- ions. This means that the concentration of hydroxide ions in the solution is the same as the concentration of sodium hydroxide, which is 0.235 M. Using the formula pOH = -log[OH-], we can find that the pOH of the solution is 0.63. This means that the concentration of hydroxide ions in the solution is 10^-0.63 M, or approximately 0.199 M.
learn more about hydroxide ions Refer: https://brainly.com/question/14576028
#SPJ11
Mr. Wang works in a recycling center. Recyclable materials arrive at the center mixed. Workers use magnets to separate steel cans from other items. Which two statements are true about the force between a steel can and a magnet?
Answers
Answer:
Option 3, The attraction between the can and the magnet is a pull.
Explanation:
The complete question is
Mr. Wang works in a recycling center. Recyclable materials arrive at the
center mixed together. Workers use magnets to separate steel cans from
other items. Which two statements are true about the force between a steel can and a magnet?
1 Gravity pushes the can toward the magnet.
2 The force between the can and the magnet is a noncontact force.
3 The attraction between the can and the magnet is a pull.
4 The attraction between the can and the magnet is a push
Solution
The force exerted by magnet on steel is the pull force. In magnets unlike poles attract each other (pull force) while the like poles repel (push force). Now, the steel or any ferrous object in the garbage when experience magnetic field develop magnetic field of their own in such a way that their north always faces the south of the external magnet or vice versa.
Hence, the force between a steel can and a magnet is pull force
Explain why light with a higher frequency would have higher energy?
Answers
Answer:
The higher the frequency, the more energy the photon has. Of course, a beam of light has many photons. This means that really intense red light (lots of photons, with slightly lower energy) can carry more power to a given area than less intense blue light (fewer photons with higher energy)
Explanation:
:)
Why is water called.compound
Answers
Answer:
because it's composed of two elements, hydrogen and oxygen. water is technically a molecular compound tho, for it has two hydrogens.
3.4 g of AGno3 are dissolved to make a 200 ml solution what is the molarity
Answers
Answer:
hehe you might think im crazy but this answer makes no sEnSe
Explanation:
Lipids that contain a high number of double bonds in their fatty acid chains will a.have a higher melting temperature than lipids that contain few double bonds in their fatty acid chains b.pack very tightly together at room temperature
c.have more carbons that rotate freely than lipids that contain few double bonds in their fatty acid chains d.likely be liquid at room temperature e.contain more hydrogens than lipids that contain few double bonds in their fatty acid
Answers
At normal temperature, lipids with a lot of double bonds in their fatty acid chains will probably be liquid.
What will happen if double bonds are present in the fatty acids?The fatty acid is referred to as being unsaturated when the hydrocarbon chain contains a double bond because it now has fewer hydrogens. A fatty acid is monounsaturated if it contains just one double bond.
Do lipids with double bonds raise melting point?The intermolecular interactions get even less as the number of cis bonds rises. The melting point decreases as a result. Hence, the melting point is actually being lowered by the double bonds. As a result, the melting point of unsaturated fatty acids drops as the number of double bonds rises.
To know more about lipids visit:-
https://brainly.com/question/30667790
#SPJ1
what is the main difference between the radiation emitted by a heated solid and the radiation emitted by an atomic gas
Answers
Color spectrum in a heated solid. The primary difference between heated solids and atomic gas is that atomic gas is not continuous (you cannot see all colors).
Which colors make up the color spectrum?unique noun When light travels through a glass prism or a drop of water, a variety of colors are created, which is what is known as the spectrum. Spectrum colors are displayed by a rainbow.
Uncomplicated definition of an emission spectrum.
The range or wavelengths that an atom or compound emits when stimulated by heat or an electric current is referred to as an emission spectrum in the field of chemistry. Every element has a different emission spectrum. The spectrum of emissions from burning fuel or even other molecules can also be used as an illustration of the substance's makeup.
To know more about spectrum visit:
https://brainly.com/question/27268130
#SPJ4
2500m into kilometer
Answers
1 meter = 1000 km
2500 meter = 2500/1000 km
= 2.5 km
Choose the transition metal among the following which has only single ionic charge ?
A. Silver (Ag)
B. Chromium (Cr)
C. Iron (Fe)
D. Copper (Cu)
Please tell what is the answer and if possible explain me...
Answers
Answer:
Silver (Ag)
Explanation:
The electronic configuration of copper is shown below;
[Kr]4d10 5s1
We can see that there is only one 5s1 electron. Hence Ag^+ tends to display a pseudo noble gas configuration. This pseudo noble gas configuration explains why silver is prevalent in the +1 oxidation state.
The other transition metals have many stable oxidation states found in nature. Chromium is observed both in +3 and +6 oxidation states. Iron is found in +2 and +3 oxidation states and copper is mostly stable in the +2 oxidation state since the +1 oxidation state readily disproportionate.
Hence silver tends to have only a single ionic charge for reasons aptly stated above.
2.00 grams of naoh is added to 100.0 ml of waer at 22.0 c. after dissolving, the temperature of the solution is found to be 27.3 c. find the experimental value for the molar enthalpy of dissolution for naoh
Answers
The 2.00 grams of NaOH is added to 100.0 mL of water at 22 °C. after dissolving, the temperature increases to 27.3 °C. The molar enthalpy of dissolution for NaOH is - 45.5 kJ/mol.
Given that :
Mass of NaOH = 2 g
Volume = 100 mL
ΔT = 5.3 °C
Mass of water = 1 × 100
= 100 g
Mass of the solution = mass of water + mass of NaOH
= 100 + 2
= 102 g
Q solution = 102 × 4.184 × 5.3
Q = 2261.8 J
Q process + Q solution = 0
Q process = - 2261.8 J
Moles of NaOH = mass / molar mass
= 2 / 40
= 0.05 mol
The molar enthalpy , ΔH = Q / n
ΔH = - 2261.8 / 0.05
= - 45236 J / mol
= - 45.4 kJ/mol
To learn more about molar enthalpy here
https://brainly.com/question/29254724
#SPJ4