Answers
Related Questions
Which of the following is NOT a sign of a chemical change? (HINT: only 2 answers, the rest are chemical change)
1. tarnished is formed
2. gas is produced
3. weight change
4. Temperature change
5. color change
6. nothing changes
7. forms a precipitate
8. energy is released
Need this done today please!!
Answers
Answer:
3 and 6 (weight change and nothing changes)
Explanation:
Hi there!
A chemical change is a change when matter changes into a new substance and has a new chemical property.
The signs that a chemical change is taking place are:
1. change in color
2. change in smell
3. change in energy (for example, there is a change in temperature (thermal energy))
4. a gas is formed (fizzing/bubbling/foaming are signs of this!)
5. formation of a solid (a precipitate)
A physical change is any change to the size, shape, or state of a substance. The substance still has the same chemical property.
these are your eight options:
1. tarnished is formed (a sign of a chemical change; a change in color)
2. gas is produced (a sign of a chemical change)
3. weight change (NOT a sign of a chemical change. Even though the weight changed, the substance still has the same chemical composition and therefore, there wasn't any change to its chemical identity)
4. Temperature change (a sign of a chemical change; a change in energy)
5. color change (a sign of a chemical change)
6. nothing changes (NOT a sign of a chemical change. If nothing happens, the substance is still the same as it was originally and there was no change to its chemical identity.)
7. forms a precipitate (a sign of a chemical change)
8. energy is released (a sign of a chemical change)
therefore the two options that are NOT a sign of a chemical change are 3 and 6 (weight change and nothing changes)
Hope this helps! :)
what does convergent plates mean
Answers
Answer:
A tectonic boundary where two plates are moving toward each other. If the two plates are of equal density, they usually push up against each other, forming a mountain chain. If they are of unequal density, one plate usually sinks beneath the other in a subduction zone. The western coast of South America and the Himalayan Mountains are convergent plate boundaries. Also called active margin collision zone See more at tectonic boundary. Compare divergent plate boundary.
What is the chemical name for F5N3
Answers
Answer:
Fluorine azide or triazadienyl fluoride (FN3) is a yellow green gas composed of nitrogen and fluorine with formula FN3. It is counted as an interhalogen compound, as the azide functional group is termed a pseudohalogen. It resembles ClN3, BrN3, and IN3 in this respect.
Explanation:
Answer:
I think it is Fluorine not sure tho
Explanation:
list the following compounds in decreasing electronegativity difference. br2 hbr kbr
Answers
The compounds listed in decreasing electronegativity difference are as follows: HBr (hydrogen bromide), KBr (potassium bromide), Br₂ (dibromine)
What is Electronegativity?
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. The greater the electronegativity difference between two atoms, the more polar the bond between them.
In the given compounds, we compare the electronegativity difference between the central atom (or atoms) and the bromine atom:
HBr: Hydrogen (H) has an electronegativity of 2.20, and bromine (Br) has an electronegativity of 2.96. The electronegativity difference is 2.96 - 2.20 = 0.76.
KBr: Potassium (K) has an electronegativity of 0.82, and bromine (Br) has an electronegativity of 2.96. The electronegativity difference is 2.96 - 0.82 = 2.14.
Br₂: Since it is a diatomic molecule, both bromine atoms have the same electronegativity of 2.96. Therefore, the electronegativity difference is 2.96 - 2.96 = 0.
Ranking the compounds based on their electronegativity differences, we have: HBr > KBr > Br₂. This means that HBr has the highest electronegativity difference, followed by KBr, and Br₂ has the lowest electronegativity difference.
To know more about Electronegativity, refer here:
https://brainly.com/question/16869675#
#SPJ4
Complete question:
list the following compounds in decreasing electronegativity difference. br₂ hbr kbr
Select the correct answer.
In an experiment, Lydia added 50 grams of sugar to 200 milliliters of water. She stirred the mixture, and the sugar eventually dissolved into the
water and couldn't be seen. The volume of the solution increased, but there was no noticeable change in color, odor, or temperature. Which
statement best describes what happened in Lydia's experiment?
Answers
Answer:
A physical change has taken place in the experiment.
A physical change is one in which there is no change in the composition of the substance. There is no change in the temperature, color or odor of the system.
In the experiment, 50 grams of sugar was added to 200 milliliters of water, upon stirring the system, the volume of the solution increased, but there was no noticeable change in color, odor, or temperature. This leads us to the conclusion that a physical change has taken place.
Explanation:
17)
Which two particles are found in the center of an atom?
-))
A)
electrons and ions
B)
protons and Deutrons
9
4
C)
protons and electrons
s
D)
electrons and neutrons
Answers
Answer:
protons and neutrons
Explanation:
this is right answer
A solution of HCl has a concentration of 3.4 x 10-3 M. What would be the [OH-] of this solution?
Answers
Answer:
a
Explanation:
uffvyffvfvf
What is the molarity of a solution composed of 6.25 g of HCl in 0.300 L ofsolution?
Answers
The concentration is measured by molarity, the formula of molarity is:
\(\text{Molarity (M)=}\frac{mole\text{s of solute}}{liters\text{ of solution}}=\frac{mol}{L}.\)Based on the given data, we've already had the volume in liters and we have to convert 6.25 grams of HCl to moles. We have to use the molar mass of HCl which is 36.4 g/mol (you can find the molar mass using the periodic table). The conversion would be:
\(6.25\text{ g HCl}\cdot\frac{1\text{ mol HCl}}{36.4\text{ g HCl}}=0.172\text{ moles HCl.}\)And the final step is to replace the values we have in the formula of molarity:
\(\text{Molarity}=\frac{0.172\text{ moles}}{0.300\text{ L}}=\text{0}.573\text{ M.}\)The molarity of 6.25 g of HCl in 0.300 L of solution is 0.573 M.
air pressure of a volleyball is 0.268atm. Pa?
Answers
Air pressure of a volleyball is 0.268atm. In Pascals it is 27132 Pa.
Air pressure is commonly measured in atmospheres (atm) or Pascals (Pa). In this case, the given air pressure of the volleyball is stated as 0.268 atm. To convert atm to Pascal, we use the conversion factor:
1 atm = 101325 Pa. Therefore, the air pressure of a volleyball, which is 0.268 atm, is: 0.268 atm x 101325 Pa/atm = 27132.06 Pa. So the air pressure of the volleyball is approximately 27132 Pa.
To know more about air pressure, click herehttps://brainly.com/question/7381033
#SPJ11
describe how to seperate a soluble solid from an insoluble liquid
Answers
Answer:
Separating solids from liquids – filtration
Filtration is a method for separating an insoluble solid from a liquid. When a mixture of sand and water is filtered: the water passes through the filter paper (it becomes the filtrate )
\(The \: techniques \: of \: dissolving, \\ filtration \: and \: evaporation \: are \: used. \\ The \: mixture \: of \: the \\ two \: solids \: is \: added \\ to \: water. \: The \: soluble \: solid \\ is \: allowed \: to \: dissolve \: and \\ the \: insoluble \: solid \: is \: filtered \\ out. \: The \: soluble \: solid \: can \\ then \: be \: obtained \: by \: evaporating \\ the \: water.\)
In period 2, ionization energy decreases as you move from Be to B, then again from N to O. The same thing happens in period three and again in period 4. How can you account for these discrepancies
Answers
The discrepancies in ionization energy within periods 2, 3, and 4 can be accounted for by considering the electron configuration and the concept of effective nuclear charge.
It depends on factors such as the nuclear charge (proton number) and the shielding effect of inner electrons.
In period 2 (from Be to O), the general trend is that ionization energy increases as you move across the period from left to right. However, there are exceptions at B and O, where the ionization energy is lower than expected.
The discrepancies can be explained by the electron configuration and the concept of effective nuclear charge. At B, there is a half-filled p orbital (2p1), which provides additional stability and makes it easier to remove an electron, resulting in a lower ionization energy compared to Be. Similarly, at O, there is a full p orbital (2p4), which again adds stability and lowers the ionization energy compared to N.
In period 3 and 4, similar trends can be observed. The discrepancies in ionization energy can be attributed to electron configurations that result in increased stability and easier removal of electrons.
The discrepancies in ionization energy within periods 2, 3, and 4 can be explained by considering the electron configuration and the concept of effective nuclear charge. The presence of stable electron configurations, such as half-filled or filled orbitals, can lead to lower ionization energy values compared to neighboring elements. This understanding helps explain the observed trends in ionization energy and highlights the importance of electron configuration in determining the physical properties of elements.
To know more about electron configuration, visit:
https://brainly.com/question/26084288
#SPJ11
which of the following options correctly describe the inscribed polygon method for predicting aromaticity? select all that apply. multiple select question. place the polygon with one of its flat sides down and draw a circle that touches all the vertices. mos below the center of the circle are bonding orbitals, and mos above the center are antibonding orbitals. where each vertex touches the circle, draw a line corresponding to a molecular orbital. aromatic systems have all bonding mos and homos completely filled with no unpaired electrons. place one electron in each bonding orbital, then pair up.
Answers
The inscribed polygon method for predicting aromaticity involves placing a polygon with one of its flat sides down and drawing a circle that touches all the vertices.
Molecular orbitals (MOS) below the center of the circle are bonding orbitals, and MOS above the center are antibonding orbitals. At each vertex where the polygon touches the circle, a line is drawn corresponding to a molecular orbital. Aromatic systems have all bonding MOS and homos (highest occupied molecular orbitals) completely filled with no unpaired electrons. This is done by placing one electron in each bonding orbital, then pairing up.
Learn more about Molecular orbitals: https://brainly.com/question/29750386
#SPJ11
The following are the correct options that describe the inscribed polygon method for predicting aromaticity:
Place the polygon with one of its flat sides down and draw a circle that touches all the vertices.Where each vertex touches the circle, draw a line corresponding to a molecular orbital.The MOS below the center of the circle are bonding orbitals, and MOS above the center are antibonding orbitals.Aromatic systems have all bonding MOs and HOMOs completely filled with no unpaired electrons.Place one electron in each bonding orbital, then pair up.The inscribed polygon method is a popular approach for predicting the aromaticity of a compound. The polygon's vertices represent the atoms of the compound, and the bond orbitals are represented by the lines. It is a cyclic arrangement of atoms with alternating single and double bonds that are more stable than their nonaromatic counterparts due to electronic delocalization. Aromaticity is a significant concept in organic chemistry because of its importance in the properties and reactivity of many organic molecules.
Learn more about aromaticity here: https://brainly.com/question/29497803
#SPJ11
How many particles are in one mole of copper (II) sulfate, CuSO4?

Answers
75. 0 mL of. 20 M CaSO4 solution are mixed with 45. 0 mL of. 35 M Li3PO4 solution. Determine the theoretical yield of Ca3(PO)42, and identify the limiting reagent
Answers
When 75.0 mL of 0.20 M CaSO4 solution is mixed with 45.0 mL of 0.35 M Li3PO4 solution, we can determine the theoretical yield of Ca3(PO4)2 and identify the limiting reagent using the principles of stoichiometry and the concept of limiting reactants.
To determine the theoretical yield of Ca3(PO4)2 and identify the limiting reagent, we need to compare the number of moles of CaSO4 and Li3PO4 present in the given solutions. First, we can calculate the number of moles for each reactant by multiplying the volume (in liters) of each solution by its respective molarity. Next, we can write the balanced chemical equation for the reaction between CaSO4 and Li3PO4 to determine the stoichiometric ratio between the reactants and the product.
By comparing the moles of CaSO4 and Li3PO4 and considering the stoichiometric ratio, we can identify the limiting reagent. The limiting reagent is the reactant that is completely consumed and determines the maximum amount of product that can be formed. The reactant that yields the smaller number of moles is the limiting reagent. Once the limiting reagent is determined, we can use its stoichiometric ratio to calculate the theoretical yield of Ca3(PO4)2, which represents the maximum amount of product that can be obtained from the given reactants.
To learn more about stoichiometric ratio, click here:
brainly.com/question/6907332
#SPJ11
The _____ property allows the positioning of an element to the right or left of other elements.
Answers
Answer:
can u show the answer it give you for the question
The float property allows the positioning of an element to the right or left of other elements.
In the context of web development and CSS, the "float" property is used to control the positioning of elements within a container. It allows you to move an element to the left or right of its containing element, enabling other elements to flow around it.
When an element is floated, it is taken out of the normal flow of the document, allowing other elements to wrap around it. This can be particularly useful for creating layouts where you want elements to appear side by side or to create text flow around images.
The "float" property can take one of two values: "left" or "right". When an element is set to "float: left", it will move to the left of its containing element, and any subsequent elements will wrap around it on the right side. Conversely, when set to "float: right", the element will move to the right, and subsequent elements will wrap around it on the left side.
Learn more about web development from the link given below.
https://brainly.com/question/30403203
#SPJ2
Have you ever tried eating delicious delicacies served in your school canteen during recess time? Have you ever thought of how these foods were prepared in such a way that various ingredients were mixed to make it delicious and healthy? The combination of several components or elements produce a useful end product that can be utilized and consumed for our advantage.

Answers
Yes, I have tried eating delicious delicacies served in the school canteen during recess time.
The food that is served in the school canteen is either cooked or in raw form.For example, I ate a vegetable sandwich and a banana smoothie for lunch. Cucumber and tomato are the sandwich's ingredients. We had bread, butter, salt, cheese, and cabbage. Bananas, milk, and sugar are all components of the banana milkshake. the cucumber has vitamin C. While tomatoes also contain vitamin C, cheese has lipids, and Banana has carbohydrates and these all components are good for health.Energy is what carbs are mostly used for, but they also aid to control blood sugar levels. While proteins are the building blocks of the body, stable lipids primarily aid in the storage of energy and the communication of information to cell membranes.The combination of these components in food produces different end products like vitamins, minerals, and many other nutrients which are beneficial for a healthy body.
Learn more about vitamins here
https://brainly.com/question/27585440
#SPJ9
What is the ph at the equivalence point in the titration of a 25.6 ml sample of a 0.397 m aqueous hydrofluoric acid solution with a 0.406 m aqueous potassium hydroxide solution?
Answers
In the titration of a 25.6 ml sample of a 0.397 m aqueous hydrofluoric acid solution with a 0.406 m aqueous potassium hydroxide solution, the pH at the equivalence point is 8.21.
Given volume of sample (V) = 25.6ml = 0.0256L
Concentration of aqueous hydrofluoric acid solution (M1) = 0.397M
Concentration of aqueous potassium hydroxide solution (M2) = 0.406M
The concentration of fluoride ions will determine the pH at the equivalency point. During the titration, the reaction is
HF + KOH --> H2O + KF.
Since it is the conjugate base of a weak acid, the F- will have an impact on the pH of the solution rather than the K+ in solution.
F- + H2O <--> HF + OH- is the reaction at the equivalence point, and the fluoride ion's molarity is 0.0256 x 0.397 = 0.010 moles of HF = moles of KOH at the equivalence point = 0.010/0.406 = 0.0246L of KOH
At the equivalence point, the F-'s molarity will be equal to = 0.010/(0.0256+0.0246) = 0.199M
Additionally, the Kb for F- is required, and its values are as follows:
Kw/Ka = 1.0 x 10-14 / (6.6 x 10-4) = 1.5 x 10-11
pOH = -log (1.8 x 10-6) = 5.79
pH = 14-5.79 = 8.21
To learn more about pH click here https://brainly.com/question/15289741
#SPJ4
Consider the compound Al(OH)3. What type of solid does it form?
Answers
Answer: crystal lattice
Explanation:
Answer:
A and the next question is c
Explanation:
Edge 2020
How many significant figures are in 15200 mL
Answers
Answer:
3
Explanation:
The zeros after 152 are not significant.
the original solution used to make the solutions for the standard curve was prepared by dissolving of (molar mass ) in enough water to make of solution. what is the molar concentration of the solution?
Answers
The original solution used to make the solutions for the standard curve was prepared by dissolving of 2.60g of CoCl₂(molar mass 130. g/mol) in water to make 100 mL of solution. The value of a concentration of the solution is 0.2 M.
The molarity is calculated as
Molarity=moles/volume(in L)
The number of moles for CoCl₂ is
moles=2.60 g×(1 mol/130. g)
moles=0.02 mol
The volume of a solution is 100. mL. Convert it into a litre
volume=100 mL×(1 L/1000 mL)=0.1 L
Plug all values in the formula
Molarity=(0.02 mol/0.1 L)
Molarity=0.2 mol/L
Molarity=0.2 M (∵M=mol/L)
Therefore, the concentration of a solution is 0.2 M.
Your question is incomplete but the complete question is
The original solution used to make the solutions for the standard curve was prepared by dissolving of 2.60g of CoCl₂(molar mass 130. g/mol) in enough water to make 100 mL of solution. What is the molar concentration of the solution?
To know more about molarity
https://brainly.com/question/19539305
#SPJ4
In mass-volume and volume-mass calculations, all gases are
considered to be at standard
____
and pressure, also
referred to as
___
answer: temperature, stp
:P
Answers
Answer: temperature, Stp
Explanation:
The calculation for standard temperature and pressure has been referred to as STP.
The mass-volume and volume-mass, has been the measurement of the concentration of the solution.
The mass-volume has been the measurement of mass of sample in the volume of solution. The volume by mass has been the measurement of the volume of solute to mass of solution.
Standard conditionsThe measurement has been performed with the standard conditions of temperature and pressure.
The standard temperature has been 273.5 K and standard pressure has been 1 atm. These temperatures and pressure has been referred to as STP.
Thus, the calculation for standard temperature and pressure has been referred to as STP.
Learn more about standard conditions, here:
https://brainly.com/question/2142674
Identify the products formed in this Brønsted-Lowry reaction. HSO−4+HF↽−−⇀acid+base
acid:
base:
Answers
In the given Bronsted-Lowry reaction between \(HSO^-^4\) and HF, the acid and base can be identified. The acid is \(HSO^-^4\), while the base is HF.
In this reaction, \(HSO^-^4\) acts as the acid because it donates a proton (\(H^+\)) to the base, HF. The \(HSO^-^4\) ion, also known as the bisulfate ion, has a hydrogen ion (\(H^+\)) that can be transferred to the base, making it an acid. On the other hand, HF acts as the base as it accepts the proton from \(HSO^-^4\). The fluoride ion (\(F^-\)) in HF has a lone pair of electrons that can accept the donated proton, resulting in the formation of the acid-base conjugate pair.
The reaction can be represented as follows:
\(HSO^-^4 + HF\) ⇌ \(H_2SO_4 + F^-\)
In this reaction, \(H_2SO_4\) is the resulting acid formed when \(HSO^-^4\) donates its proton to HF, which becomes the resulting base in the form of fluoride ion (\(F^-\)). The reaction is reversible, indicating that the acid and base can interconvert depending on the reaction conditions.
Learn more about Bronsted-Lowry reaction here:
https://brainly.com/question/31659921
#SPJ11
At a certain temperature, the equilibrium constant K for the following reaction is 819.: Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.51 mole of CO_2 and 0.51 mole of H_2. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. There will be very little CO and H_2O. There will be very little CO_2 and H_2. Neither of the above is true.
Answers
At equilibrium, the composition of the reaction vessel with 0.51 moles of CO₂ and 0.51 moles of H₂ is given by the equilibrium constant K, which is 819.
The reaction given is:
H₂(g) + CO₂(g) ⇌ CO(g) + H₂O(g)
So, we know the formula for Kp and Kc in terms of partial pressure and concentration are as follows:
Kc = (CO(g) × H₂O (g)) / (CO₂(g) × H₂(g))
Kp = (PCO × PH₂O) / (PCO₂ × PH₂)
At equilibrium, the equilibrium constant expression can be written as:
Kc = [CO][H₂O] / [H₂][CO₂] = x²/ [(0.51-x)(0.51-x)]
Substituting the given value of Kc into the above equation and solving for x gives:
819 = x²/[(0.51-x)(0.51-x)]
x = 0.302
From the results, we can say that "There will be very little CO₂ and H₂" at equilibrium.
The equilibrium constant for the given reaction is 819.
Learn more about equilibrium constants here:
https://brainly.com/question/3159758
#SPJ11
a compound was analyzed and found to contain 13.5 grams calcium, 10.8 grams oxygen, and 0.675 grams of hydrogen. what is the empirical formula for the compound?
Answers
The empirical formula for the compound is Ca(OH)₂.
To determine the empirical formula of the compound, we need to find the simplest whole-number ratio of the elements present. We can do this by calculating the number of moles of each element and then finding the ratio between them.
Mass of calcium (Ca) = 13.5 grams
Mass of oxygen (O) = 10.8 grams
Mass of hydrogen (H) = 0.675 grams
First, we need to convert the masses of each element to moles using their respective atomic masses.
Atomic mass of Ca = 40.08 g/mol
Atomic mass of O = 16.00 g/mol
Atomic mass of H = 1.01 g/mol
Number of moles of Ca = 13.5 g / 40.08 g/mol = 0.3367 mol
Number of moles of O = 10.8 g / 16.00 g/mol = 0.675 mol
Number of moles of H = 0.675 g / 1.01 g/mol = 0.6693 mol
Next, we need to find the simplest whole-number ratio by dividing the number of moles of each element by the smallest number of moles obtained.
Dividing by 0.3367 (smallest value):
Number of moles of Ca = 0.3367 mol / 0.3367 mol = 1
Number of moles of O = 0.675 mol / 0.3367 mol = 2.005
Number of moles of H = 0.6693 mol / 0.3367 mol = 1.988
Rounding these values to the nearest whole number, we have:
Number of moles of Ca = 1
Number of moles of O = 2
Number of moles of H = 2
Thus, the empirical formula of the compound is Ca(OH)₂.
Learn more about empirical formulas at https://brainly.com/question/13058832
#SPJ11
I NEED HELP ASAPPP PLZ

Answers
1. Heat needed = 293.25 J
2. Time to leave : 2 s
Further explanationGiven
1.5 g H2
Cp H2 = 14.27 g/J C
Δt= 13.7
Required
1. Heat needed
2. Fridge time
Solution
1. Heat can be formulated :
Q = m.c.Δt
Q = 1.5 x 14.27 x 13.7
Q = 293.25 J
2. Heat decreases = 150 J/s, so for 293.25 J :
\(\tt t=\dfrac{293.25~J}{150~J/s}=1.955~s\approx 2~s\)
The equilibrium constant of a reaction requires certain environmental variables to remain constant. These variables are _____.
pressure, temperature, and concentration
temperature and concentration
pressure, temperature, and time
None of the above.
Answers
The equilibrium constant of a reaction requires certain environmental variables to remain constant. These variables are pressure, temperature, and concentration. The correct option is A.
An equilibrium constant is a mathematical tool that enables the quantification of the extent of a chemical reaction. The equilibrium constant is symbolized by Keq, and it is utilized to determine the concentration of reactants and products present at equilibrium.
This calculation is done using the law of mass action.Keq is defined as the ratio of product concentrations to reactant concentrations in a chemical reaction taking place at equilibrium. The concentrations used in the expression for Keq are equilibrium concentrations.
As a result, Keq is a constant for a given reaction at a specific temperature. Keq is dependent on a variety of environmental variables such as temperature, pressure, and concentration. To keep the equilibrium constant stable, these variables must remain constant.
Learn more about equilibrium constant
brainly.com/question/28559466
#SPJ11
Jana is modeling mutations using the word "FRIEND."
Which version of the word "FRIEND' models a deletion mutation?
A FRIENDS
B. DRIEND
C. FIEND
D. DNEIRF
Answers
Answer:
It is c I am doing the test I hope this helps ; )
Explanation:
Answer:
c
Explanation:
which molecule would be linear? (in each case you should write a lewis structure before deciding.) a) so2 b) hcn c) h2o2 d) h2s e) of2
Answers
The correct option is e) OF2
A molecule is linear if all its atoms lie in a straight line. Among the given molecules, the one that would be linear is OF2.
OF2 stands for oxygen difluoride. It is a covalent compound that contains two fluorine atoms bonded to a single oxygen atom, resulting in the molecular formula OF2.
Lewis structure of OF2: Before we decide whether OF2 is linear or not, let's draw the Lewis structure of the molecule:
VSEPR theory is used to predict the geometry and shape of molecules. According to the VSEPR theory, electron pairs in the valence shell of the central atom of a molecule repel each other and arrange themselves to be as far apart as possible to minimize repulsion forces.The geometry of a molecule is determined by the total number of electron pairs around the central atom of the molecule, which is called the steric number. The shape of the molecule is determined by the arrangement of these electron pairs.For OF2, the steric number of the central atom (oxygen) is three. Therefore, according to VSEPR theory, the molecular geometry of OF2 is V-shaped or bent. However, the molecule is linear with respect to the central atom (oxygen) because there are no lone pairs on oxygen atom, but only two bonding pairs, which are directed opposite to each other. In conclusion, the molecule that is linear among the given molecules is OF2.
Learn more about Lewis structure of OF2
https://brainly.com/question/32550284
#SPJ11
why is the temperature at which density is measured usually specified
Answers
Density is a crucial physical quantity for a variety of materials, including liquids, gases, and solids. The temperature at which density is measured is usually specified because density is temperature-dependent and changes with temperature.
This implies that a small change in temperature will have a substantial effect on density.Let's have a look at the connection between temperature and density:T and ρ are the symbols used to represent temperature and density, respectively. It is common knowledge that substances expand when heated and contract when cooled. In the same vein, as temperature increases, the space between the molecules increases, resulting in a decrease in density. Conversely, if the temperature decreases, the molecules move closer together, and the density increases. When we consider the relationship between temperature and density, we can infer that density is directly proportional to temperature. As a result, when the temperature changes, so does the density, and this fluctuation must be specified. Therefore, specifying the temperature at which density is measured ensures accuracy and consistency in measurements. It also allows us to make appropriate comparisons between density values obtained from various sources. In conclusion, specifying temperature when measuring density ensures consistency and accuracy in results.
To know more about Density visit :
brainly.com/question/29775886
#SPJ11
Write a balanced equation for the following nuclear decay reaction.
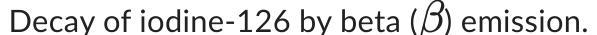
Answers
The balanced equation for the beta emission decay of iodine-126 is as follows:
Iodine-126 (126I) → Xenon-126 (126Xe) + Beta particle (β-)What is decay process in nuclear reaction?Decay process in nuclear reactions refers to the spontaneous transformation of an unstable nucleus into a more stable one, emitting one or more particles or radiation in the process.
The types of decay include
alpha decay, beta decay, and gamma decay.This process results in a decrease in the total number of protons and neutrons in the nucleus and a decrease in the total atomic number and mass number of the nucleus.
In the equation, the beta particle is an electron emitted from the nucleus during the decay process.
Learn more about beta emission at:
https://brainly.com/question/16409346
#SPJ1
