List three organelles found in cells and describe their functions.
Answers
nucleus: controls cell
cell membrane: controls what goes in and out of a cell
cytoplasm: where chemical reactions take place
Answer:
Nucleus: DNA Storage
Mitochondrion Energy production
smooth Endoplasmic Reticulum (SER) Lipid production; Detoxification
Related Questions
The original concentration in a sample is 2.79 x 10^6 cfu/ml. which sample volume should yield a countable plate (i.e., between 30-300)?
Answers
To get a countable plate, you need to dilute the sample. A 1:10 or 1:100 dilution will likely yield a countable plate.
When a sample has a high concentration of bacteria, it can be challenging to get a countable plate as the number of colonies can be too numerous to count accurately. To address this issue, the sample needs to be diluted. A 1:10 or 1:100 dilution is commonly used to reduce the bacterial concentration to a countable range.
To calculate the dilution factor, you divide the original concentration by the dilution factor used. For example, a 1:10 dilution would be 10^-1, and a 1:100 dilution would be 10^-2. To achieve a concentration between 30-300 cfu/ml, you need to calculate the volume of the diluted sample needed to plate out.
For instance, a 1:100 dilution of the original sample would result in a concentration of 2.79 x 10^4 cfu/ml. To plate out between 30-300 colonies, you would need to plate between 0.1 to 1 ml of the diluted sample.
Learn more about dilution here:
https://brainly.com/question/28548168
#SPJ11
Jose and Sue were investigating the formation of precipitates in chemistry lab. They
mixed a silver nitrate solution with a sodium chloride solution and immediately a
white solid appeared in the bottom of the test tube. This white solid is a precipitate
of silver chloride. The reaction is represented with the equation:
AgNO3(aq) + NaCl (aq) → AgCl (s) + NaNO3(aq)
Answers
The given reaction of silver nitrate with sodium chloride is an example of double displacement reaction where, two groups replaces each other. Thus, option D is correct.
What is double displacement reaction?Displacement reaction is a type of reaction in which a species from a reactant is displaced by other species or group from reagent. There are single displacement reaction as well as double displacement reaction.
In single displacement reaction, only one group is displaced by another group from the second reactant. Whereas in double displacement reaction, two groups are displaced each other between two reactants.
In the reaction between silver nitrate and sodium chloride, the nitrate group and chloride group interchange between the metals silver and sodium as written in the reaction. Hence, it is a double displacement reaction.
To find more on displacement reaction, refer here:
https://brainly.com/question/29307794
#SPJ1
Your question is incomplete, but your complete question probably was:
Jose and Sue were investigating the formation of precipitates in chemistry lab. They
mixed a silver nitrate solution with a sodium chloride solution and immediately a
white solid appeared in the bottom of the test tube. This white solid is a precipitate
of silver chloride. The reaction is represented with the equation:
AgNO3(aq) + NaCl (aq) → AgCl (s) + NaNO3(aq)
The equation represents a ________________ reaction.
A) synthesis
B) decomposition
C) neutralization
D) double replacement
WILL GIVE BRAINLIEST!!!
What is the mass of NaCl required to make 160 grams of a 32% solution of NaCl in water?
Answers
Answer:
19.20 g. Explanation: ∵ mass % = [mass of solute/mass of solution] x 100
Can someone help me

Answers
Answer:the second largest one
Explanation:
both weels are pushing at it
Which sentence would be inappropriate in an informational piece of writing with a formal tone?
A: People find Shakespeare's Pericles, Prince of Tyre boring and annoying because it's possible that he didn't even write the entire play.
B: Thirty-nine plays, a combination of comedies, histories, and tragedies, are attributed to William Shakespeare.
C: William Shakespeare, a poet, and playwright have generally deemed the greatest writer in the history of the English language.
D: Scholars speculate that Hamlet may have been informed by the relationship between Shakespeare and his son Hamnet, who died at eleven years of age.
Answers
Answer:
Its a
Explanation:
i took the quiz
The sentence that is inappropriate in an informational piece of writing with a formal tone is "People find Shakespeare's Pericles, Prince of Tyre boring and annoying because it's possible that he didn't even write the entire play." The correct option is A.
What is a formal tone?In academic or professional settings, a formal writing tone is common. This tone emphasizes thoroughness and directness while remaining respectful.
It emphasizes facts and grammatical correctness and uses full words rather than contractions.
The use of standard English, more complex sentence structures, the infrequent use of personal pronouns, and the absence of colloquial or slang terms characterize formal language.
In informal language, nonstandard English forms, colloquial vocabulary, and typically shorter sentence structures are permitted.
"People find Shakespeare's Pericles, Prince of Tyre boring and annoying because it's possible that he didn't even write the entire play," is an inappropriate sentence in a formal informational piece of writing.
Thus, the correct option is A.
For more details regarding formal tone, visit:
https://brainly.com/question/14330525
#SPJ2
What single property was the most important in Jessica's material?
Jessica Kusher
Answers
Explanation: Jesseca Kusher, an 18-year-old researcher from Spartansburg, S.C., invented a paint-on coating for roofing shingles. Her formula could reduce a home's cooling costs and possibly cut ozone pollution in urban areas. ... One coating got graphite, the same material in pencil lead. Another recipe included gypsum
Forces and Motion:Question 1
A rider on a bicycle travels north for 1.5 hours and covers a distance of 18
kilometers. What is the velocity of the bicycle?
Answers
Answer:
Velocity = 12 km/h
Explanation:
Velocity can be defined as the division between distance traveled and the time it took for that distance to be traveled:
Velocity = Distance / TimeWith the above information in mind, we can calculate the velocity of the bicycle:
Velocity = 18 km / 1.5 hVelocity = 12 km/hThus the velocity of the bicycle is 12 kilometers per hour.
a
Jessica has a solution of tea and water. She adds ice to the solution.
Which properties of the solution will change as the ice melts?
a. The mass will decrease.
b. The temperature will decrease.
The volume will increase.
C. The volume will increase
Answers
if he gas has an average kinetic energy of 5450 j/mol under certain conditions, what is the root mean square speed of cl2 gas molecules under the same conditions?
Answers
The root mean square speed of Cl₂ gas under the same conditions will be 391.8 m/s if He(g) has an average KE of 5450 J/mol under certain conditions.
We know that the RMS speed of the gas is expressed as
Vrms = \(\sqrt{\frac{3RT}{M} }\)
here, T: absolute temperature of the gas
M: molar mass of the gas
R: universal gas constant
so average molar kinetic energy of the gas
E = 1/2MV²rms = (3/2)RT
Now, given that average kinetic energy for He gas
KE = 5450 J/mol
we know that molar KE is independent of the nature of the gas. It only depends on temperature as the ideal conditions are concerned. So both He and Cl₂ have same KE = 5450 J/mol
So for Cl₂
atomic mass, M = 35.5g/mol or 35.5 x 10⁻³kg/mol
(1/2MV²rms ) for Cl₂ = 5450
V² = \(\frac{2x5450}{2x35.5x10^{-3}}\)
or V = 391.8 m/s
Therefore, the root mean square speed of Cl₂ gas under the same conditions will be 391.8 m/s if He(g) has an average KE of 5450 J/mol under certain conditions.
#SPJ4
To learn more about RMS speed:
https://brainly.com/question/30097478
Which functional group does the molecule below have?
H H H H
H-Ċ-Ċ-Ċ-Ċ-0-H
| | | |
н ннн
O A. Amino
O B. Ester
O C. Ether
O D. Hydroxy
Answers
Because of the OH substituent
raffinose and stachyose are examples of what type of complex carbohydrate?
Answers
The raffinose and the stachyose are the examples of the type of complex carbohydrate is the oligosaccharides.
The oligosaccharide, are the carbohydrate of from of the three to six units of the simple sugars that is monosaccharides sugar. The classification of the oligosaccharides depends on the number of the sugar units they made up of is as follows :
TrisaccharidesTetrasaccharidesPentasaccharidesHexasaccharidesIn the trisaccharide the three monosaccharides linked together. In the tetrasaccharides the four monosaccharides that is 2 galactose units, one unit of fructose and one glucose unit. In the pentasaccharides five monosaccharides. The hexasaccharide are made up of the six sugar units.
To learn more about carbohydrates here
https://brainly.com/question/13712534
#SPJ4
Periodic table trivia crossword puzzle
Noble gases all share?
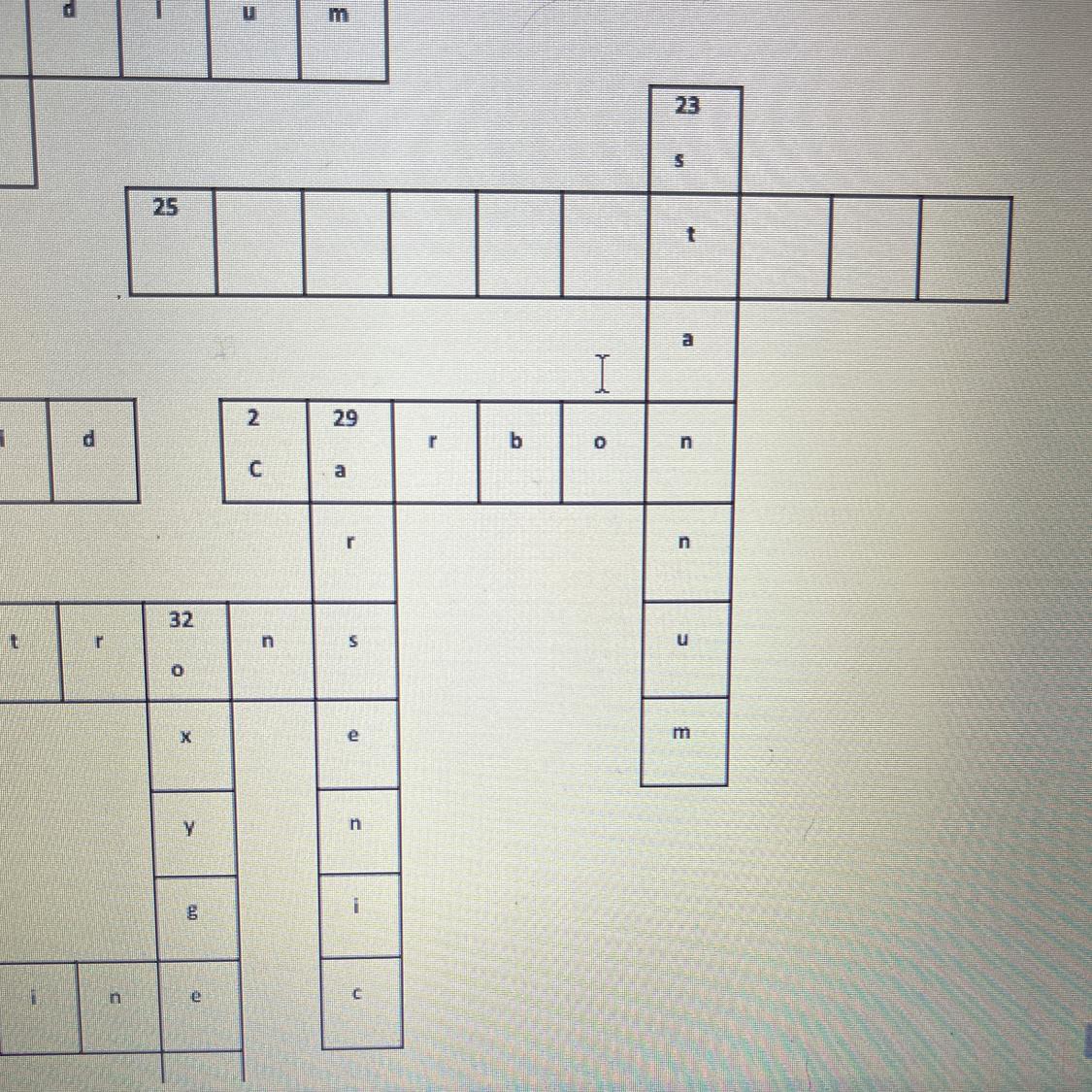
Answers
Answer:
properties
Explanation:
i think it is properties because it fits the space
explain how components of the atmosphere can be used successfully in producing important chemicals
Answers
Oxygen and nitrogen from the atmosphere can be used as feedstock to produce chemicals such as ammonia, nitric acid, and sulfuric acid.
What are chemicals?The components of the atmosphere, such as nitrogen and oxygen, can be used to produce important chemicals through industrial processes such as the Haber-Bosch process for ammonia synthesis and the production of nitric acid. Nitrogen and oxygen can also be used as oxidizers in combustion processes to produce energy and heat, such as in the burning of fossil fuels.
Additionally, carbon dioxide from the atmosphere can be used as a feedstock for the production of chemicals such as methanol and formic acid through processes like carbon capture and utilization. The use of atmospheric components in chemical production can help to reduce reliance on non-renewable resources and support the development of sustainable manufacturing processes.
Learn more about chemicals, here:
https://brainly.com/question/18234342
#SPJ2
15 g of an unknown metal cools from 50 °C to 25.5 °C and loses 220.6 J of heat. What is the specific heat of the metal in J/(g °C)?
2. You grab 200 g of rocks that have been sitting by the fire to warm your sleeping bag while camping. The rocks were initially 36 °C when you removed them from the fire and 28 °C when you woke up in the morning. You determine that 145 joules of heat were lost. What is the specific heat of those rocks?
3. A new type of medical warming pad specifically designed to improve blood flow uses a unique new compound that stores heat longer than other similar products. The medical warmer contains 6 kg of the new compound and has a specific heat of 6.76 J/(g °C). How much energy can the new compound store if it is heated from 28 °C to 40 °C?
4. The specific heat of tin is 0.117 J/(g °C). How much energy is required to heat 200 grams of tin from 30 °C to 75 °C?
5. How many kilograms of lead would it take to generate 3000 joules of energy if it was cooled from 100 °C to 40 °C? (c of Au = 0.129)
6. A new superfood was discovered that contains all the vitamins and minerals needed for normal human function. A 10-gram sample of the superfood was heated and placed into 10 g of water at 20 °C in an insulated cup calorimeter. The final temperature of the water was 18 °C. What is the heat change of the superfood? (c of water = 4.184)
7. Ethanol and ethylene glycol are combined in a calorimeter with 3.8 g of water at 17 °C. The final temperature of the water is 13 °C. What is the heat change of the reaction? Was this an endothermic or exothermic reaction? (c of water = 4.184)
8. Hydrogen peroxide and an oxalate ester, chemicals used in glow sticks, are mixed in a calorimeter with 5 g of water at 28 °C. You determine the heat change was -323 joules. What was the final temperature of the water? Was this an endothermic or exothermic reaction? (c of water = 4.184)
Answers
1.The specific heat is X J/(g °C),2.
The specific heat of rocks is X J/(g °C),
3.New compound can store X joules,
4.Energy is X joules,
7.The heat change of the reaction is X joules,
8.Final temperature is X °C.
To calculate the specific heat of the metal, we can use the formula Q = m * c * ΔT, where Q is the heat lost, m is the mass of the metal, c is the specific heat, and ΔT is the change in temperature. Rearranging the formula, we have c = Q / (m * ΔT). Plugging in the given values, we find the specific heat of the metal.
Similar to the previous question, we can use the formula Q = m * c * ΔT to calculate the specific heat of the rocks. Given the mass, heat lost, and temperature change, we can solve for c.
The energy stored in the new compound can be calculated using the formula Q = m * c * ΔT, where Q is the energy, m is the mass of the compound, c is the specific heat, and ΔT is the change in temperature. Plugging in the given values, we can determine the energy stored in the compound.
Using the formula Q = m * c * ΔT, we can calculate the energy required to heat the tin. Given the mass, specific heat, and temperature change, we can solve for Q.
It would take X kilograms of lead.
To calculate the mass of lead needed, we can use the formula Q = m * c * ΔT. Rearranging the formula, we find m = Q / (c * ΔT). Plugging in the given values, we can determine the mass of lead required.
The heat change of the superfood is X joules.
The heat change can be calculated using the formula Q = m * c * ΔT, where Q is the heat change, m is the mass of the superfood, c is the specific heat of water, and ΔT is the change in temperature of the water. By plugging in the given values, we can find the heat change of the superfood.
To calculate the heat change of the reaction, we use the formula Q = m * c * ΔT, where Q is the heat change, m is the mass of water, c is the specific heat of water, and ΔT is the change in temperature of the water. The sign of Q indicates whether the reaction is endothermic (positive) or exothermic (negative).
To find the final temperature of the water, we can use the formula Q = m * c * ΔT. Rearranging the formula, we have ΔT = Q / (m * c). Plugging in the given values for heat change, mass of water, and specific heat, we can solve for ΔT and find the final temperature of the water. The sign of the heat change (Q) determines if the reaction is endothermic (positive) or exothermic (negative).
To learn more about heat
https://brainly.com/question/25603269
#SPJ11
The questions touch on the concept of specific heat and heat transfer. By using the specific heat formula (q = mcΔT) and appropriately adjusting it to the problem at hand, one can calculate the specific heat, the stored energy, the heat changes and the final temperature in each case.
Explanation:The questions asked are in the realm of physics, particularly focusing on the concept of specific heat, heat transfer and the heat changes in various compounds and chemicals. To answer these questions, we can make use of the formula for specific heat: q = mcΔT where 'q' is the heat transfer, 'm' represents the mass, 'c' is the specific heat and 'ΔT' depicts the change in temperature.
1. Using the formula, the specific heat of the unknown metal can be calculated by rearranging the formula to c = q / (m * ΔT) = 220.6 J / (15g * 24.5°C).
2. For the rocks, you would calculate c = 145 J / (200g * 8°C).
3. The energy storage of the medical pad's compound can be determined by rearranging the formula to find q = (6kg * 6.76 J/g°C * 12°C).
4. The energy required to heat the tin can be calculated using q = (200g * 0.117 J/g°C * 45°C).
5. The mass of lead can be determined by rearranging the formula: m = q / (c * ΔT) = 3000 J / (0.129 * 60°C).
6 & 7. The heat change of the superfood and reaction in the calorimeter can also be determined using this formula.
8. The final temperature of the water can be determined by q = mcΔT announcing ΔT = q / (mc) = -323 J / (5g * 4.184 J/g°C).
Learn more about Specific Heat here:https://brainly.com/question/31608647
#SPJ2
for the neutralization reaction below in which a strong base is reacted with a strong acid lioh hcl --> h2o na cl- how many grams of lithium hydroxide are needed to completely neutralize 500 ml of a 0.2 m solution of hcl?
Answers
2.395 grams of lithium hydroxide are needed to completely neutralize 500 ml of a 0.2 m solution of HCl.
What do you mean by neutralization reaction?A chemical reaction between an acid and a base that results in a more neutral solution is known as a neutralization reaction. The strength of the acid and base used in the reaction determines the pH of the solution.
For the neutralization reaction: LiOH + HCl -> H2O + NaCl
To find the number of moles of HCl present in the solution:
0.2M * 0.5L = 0.1 moles of HCl
As the reaction is a neutralization reaction, the number of moles of HCl will be equal to the number of moles of LiOH used in the reaction.
Now, we know the number of moles of LiOH used in the reaction is 0.1 moles.
To find the mass of LiOH used in the reaction, we can use the formula: mass = moles * molar mass
The molar mass of LiOH is 23.95 g/mol.
mass = 0.1 moles * 23.95 g/mol = 2.395 g of LiOH.
Therefore, 2.395 grams of lithium hydroxide are needed to completely neutralize 500 ml of a 0.2 m solution of HCl.
To know more about neutralization reaction, visit:
brainly.com/question/27891712
#SPJ4
if 1.600 moles of a solute are dissolved in enough water to make 400.0 ml of solution, what is the molarity?
Answers
The molarity of the solution if 1.600 moles of a solute are dissolved in enough water to make 400.0 ml is 4M
Before we calculate the molarity we should know that molarity is a unit of concentration measuring the number of moles of a solute per liter of solution.
The molarity can be calculated as follows:
as we know, 400 mL = 0.4L
Molarity = moles/volume
molarity = 1.600 moles/ 0.4L
Molarity = 4M
so,The molarity of the solution if 1.600 moles of a solute are dissolved in enough water to make 400.0 ml is 4M
Find out more information about Molarity here: https://brainly.com/question/9685175
#SPJ4
Two samples of sodium chloride were decomposed into their constituent elements. One sample produced 2.84 g of sodium and 4.37 g of chlorine. Which of the following could be the results of the decomposition of the other sample, being consistent with the law of constant composition (also called the law of definite proportions or law of definite composition)?
a) 4.17 g of sodium and 3.75 g of chlorine
b) 4.17 g of sodium and 6.42 g of chlorine
c) 4.17 g of sodium and 1.05 g of chlorine
d) 4.17 g of sodium and 12.1 g of chlorine
Answers
Answer:
The correct answer is b) 4.17 g of sodium and 6.42 g of chlorine
Explanation:
According to the law of definite proportions a chemical compound is composed always by the same elements in the same proportions by mass. In this case, the proportion of the elements by mass will be 4.37 g of chlorine (Cl) per 2.84 g of sodium (Na):
4.37 Cl/2.84 g Na= 1.54
We can calculate the proportions of the results in order to see which is the correct:
a) 3.75 g Cl/4.17 g Na = 0.899
b) 6.42 g Cl/4.17 g Na = 1.539 ⇒ ≅1.54
c) 1.05 g Cl/4.17 g Na = 3.971
d) 12.1 g Cl/4.17 g Na = 2.901
The option in which the proportion Cl/Na is equal to 1.54 is option b
How many moles of elemental bromine do you expect to consume in this reaction? how many microliters of your bromine solution will this require? what temperature will your reaction mixture be as it refluxes? should you use a water condenser, or is air condensation likely to be sufficient?
bromaination of alkenes is an anitu-addituinn: i,e the substituensts attach to their respective carbons on opposite sides of th eplane of the molecule. Do they remain in opposite sides of the molecule after that? what are the absolute configuratuins of the carbons? draw rhe product to illustrate your answer
Answers
The temperature of the bromine reaction mixture during reflux, it typically depends on the boiling point of the solvent being used.
For example, if the solvent is chloroform, the reflux temperature would be around 61-62°C. If the solvent is carbon tetrachloride, the reflux temperature would be around 76-77°C.
As for the condenser, a water condenser is typically used during reflux to prevent the loss of solvent and/or reagents due to evaporation. Air condensation is not likely to be sufficient, especially for reactions that require longer reflux times.
Regarding the bromination of alkenes, the substituents do remain on opposite sides of the molecule after the reaction, resulting in a trans product. The absolute configurations of the carbons depend on the starting configuration of the alkene. For example, if the starting alkene is (Z)-2-butene, the product of bromination would be (2R,3S)-2,3-dibromobutane, as shown in the following diagram:
H Br
| |
H -- C=C -- C -- H
| |
Br H
Note that the stereochemistry of the product is determined by the anti-addition mechanism of bromination, which results in the formation of a meso compound with two chiral centers.
Learn more about bromine visit: brainly.com/question/29301746
#SPJ4
Question 4 of 25
How do scientists write very large numbers?
O A. They round off all numbers to a few digits after the decimal.
O B. They express the numbers using scientific notation.
O C. They divide all numbers by a constant to reduce the size.
O D. They simplify the numbers by deleting the exponents.
Answers
Answer:
B.
Explanation:
8. I have a mixture of iron, salt, and
water. I heat it up to a boil.
Explain what happens to each part
of my mixture.
Answers
After boiling the mixture of iron salt, water
iron, and salt it will remain and water will be evaporated
example of physical property
Answers
Answer:
Color. I thinkkk.
Phyiscal properties are defined as properties that can be measured or observed without changing the chemical nature of the substance.
Some examples of phsyical properties are:
- Color
- Density
- Volume
- Mass
- Boiling or Melting Points
how long does it take for l-citrulline to work for ed
Answers
L-citrulline is an amino acid that is commonly used as a supplement for erectile dysfunction (ED). The time it takes for L-citrulline to work can vary depending on several factors, including the individual's metabolism and the dosage taken.
Typically, it is recommended to take L-citrulline supplements daily for several weeks to see noticeable improvements in ED symptoms. However, some individuals may experience positive effects sooner, within a few days or even hours after taking the supplement.
To achieve the best results, it is important to follow the recommended dosage instructions provided by the manufacturer or a healthcare professional. It is also worth noting that L-citrulline may be more effective when combined with other supplements or medications, such as L-arginine or PDE5 inhibitors like Viagra.
As with any supplement or treatment, it is advisable to consult with a healthcare professional before starting L-citrulline or making any changes to your current treatment plan for ED. They can provide personalized advice based on your specific needs and health condition.
Learn more about healthcare professional from:
https://brainly.com/question/28467175
#SPJ11
8. Study the given table and answer the following questions. i) Name metals and non-metals. Elements ii) What is the valency of A and why? iii) Write the name and symbol of all the elements. iv) Write down the molecular formula of the compound formed by the combination of A and B; and C and B. A B C D Electronic configuration 2,8,1 2,8,7 2,8,8,2 2,8 v) Which element is more active between A and D? vi) Name the elements which can lose the valence electron to become stable.
Answers
i) Metals: A and D
Non-metals: B and C
ii) The valency of A is 1. This is because A has one valence electron, and elements in Group 1 (such as A) typically have a valency of 1 as they tend to lose that single valence electron to achieve a stable electron configuration.
iii)
A: Sodium (Na) - Symbol: Na
B: Chlorine (Cl) - Symbol: Cl
C: Oxygen (O) - Symbol: O
D: Calcium (Ca) - Symbol: Ca
iv) The compound formed by the combination of A and B: Sodium Chloride (NaCl) The compound formed by the combination of C and B: Oxygen Chloride (OCl2)
v) Element D (Calcium) is more active than element A (Sodium). This is because elements in Group 2 (such as D) tend to lose two valence electrons, which requires less energy compared to losing only one valence electron (as in the case of A).
vi) The elements that can lose the valence electron to become stable are A (Sodium) and D (Calcium).
Metals: A, B, C, D; Non-metals: None. Elementsii) The valency of A is 1 because it has only one valence electron.iii) The name and symbol of all the elements are:A - Sodium (Na)B - Chlorine (Cl)C - Argon (Ar)D - Calcium (Ca)iv) The molecular formula of the compound formed by the combination of A and B is NaCl. The molecular formula of the compound formed by the combination of C and B is BCl2.
v) A is more active than D because A is a metal and metals are more active than non-metals. A tends to lose electrons more easily than vi) The elements which can lose the valence electron to become stable are metals.
i) Metals: A and D Non-metals: B and C ii) The valency of A is 1. This is because A has one valence electron, and elements in Group 1 (such as A) typically have a valency of 1 as they tend to lose that single valence electron to achieve a stable electron configuration. iii) A: Sodium (Na) - Symbol: Na B: Chlorine (Cl) - Symbol: Cl C: Oxygen (O) - Symbol: O D: Calcium (Ca) - Symbol: Ca
iv) The compound formed by the combination of A and B: Sodium Chloride (NaCl) The compound formed by the combination of C and B: Oxygen Chloride (OCl2) v) Element D (Calcium) is more active than element A (Sodium). This is because elements in Group 2 (such as D) tend to lose two valence electrons, which requires less energy compared to losing only one valence electron (as in the case of A). vi) The elements that can lose the valence electron to become stable are A (Sodium) and D (Calcium).
for such more questions on electrons
https://brainly.com/question/26084288
#SPJ8
please answer
Classifying Unfamiliar Substances (Chemical names): Questions to be asking
1. Is it on the Periodic table?
a.Yes – Then its an element
b. No – Go to Number 2.
2. Is it an “Ide”?
a.Yes – Then it is a compound
b. No – Go to Number 3
3. Is it an “Ate”?
a.Yes – Then it is a compound
b. No – Go to Number 4
4. Is it an “Ane”?
a.Yes – Then it is a compound
b. No – Go to Number 5
5. Does it have “and” in it?
a.Yes – then it is a Mixture
b.No – then the substance could still be a Mixture
10
Substance
Classification
(eg Element)
Reason
Oxygen
Element
On periodic table
Petroleum
Hydrogen and
Helium
Iron Sulphide
Carbon Dioxide
Salt Water
Milk and Sugar
Silver Nitrate
Sodium Carbonate
Carbon Electrode
Astatine
Argon
Oxidane
Iron and Sulphur
Methane
Answers
Answer:
1) petroleum, mixture, not found on periodic table
2) hydrogen and helium, mixture, contains "and"
3) iron sulphide, compound, ends with "ide"
4) carbon dioxide, compound, ends with "ide"
5) salt water, mixture, not found on periodic table
6) milk and sugar, mixture, contains "and"
7) silver nitrate, compound, ends with "ate"
8) sodium carbonate, compound, ends with "ate"
9) carbon electrode, mixture, not found on periodic table
10) astatine, element, found on periodic table
11) argon, element, found on periodic table
12) oxidane, compound, contains "ane"
13) iron and sulphur, mixture, contains "and"
14) methane, compound, contains "ane"
Explanation:
if you use too much hot solvent when dissolving your crude compound, how will that impact the recovery of your compound and why?
Answers
Recrystalization will occur.The solution may become too diluted for crystals to form if you add too much solvent. Impurities will be captured by a hastily formed crystal's lattice. The crystals that result will also be smaller.
What is Recrystalization?Recrystallization is a physical process used to separate compounds based on how soluble they are. Heating the material to dissolve the compound with impurities in a mixture of a suitable solvent completes the procedure. We can remove the desired chemical or contaminants from the mixture using this method.
The solution may become too diluted for crystals to form if you add too much solvent. The flask needs to be gently cooled, first at room temperature and then in cold water. Impurities will be captured by a hastily formed crystal's lattice. The resulting crystals will also be smaller.
This method is used to harden steel in order to eliminate all strain hardening side effects, including the significant plastic deformation brought on by cold working.The crystals that frequently form when the compound precipitates out gave it its name. The natural expansion of larger ice crystals at the expense of smaller ones is another definition of recrystallization.Some commonly effective mixes include diethyl ether-methanol (or ethanol) for polar molecules (particularly esters, alcohols, and hydrocarbons) and diethyl ether-petroleum ether (or benzene) for strongly linked solids (notably amides, alcohols), as well as many natural products.The three main types of recrystallization are;
Single-solvent recrystallization.Multi-solvent recrystallization.Hot filtration-recrystallization.To know more about Recrystalization, refer to:
https://brainly.com/question/10194206
#SPJ1
for the previous light of 671 nm, if a light emitted 0.50 moles of this photon, what is the energy of this light?
Answers
The energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.92 * 10^4 Joules.
Let's understand this in detail:
To find the energy of light emitted by 0.50 moles of photons with a wavelength of 671 nm, we can follow these steps:
1. Convert the wavelength to meters: 671 nm * (1 meter / 1,000,000,000 nm) = 6.71 * 10^-7 meters.
2. Calculate the energy of one photon using the Planck's equation: E = hf, where E is energy, h is Planck's constant (6.626 * 10^-34 Js), and f is frequency.
3. To find the frequency, we use the speed of light (c) equation: c = λf, where λ is the wavelength. Rearrange the equation to find the frequency: f = c / λ.
4. Substitute the values and calculate the frequency: f = (3 * 10^8 m/s) / (6.71 * 10^-7 m) = 4.47 * 10^14 Hz.
5. Now, calculate the energy of one photon: E = (6.626 * 10^-34 Js) * (4.47 * 10^14 Hz) = 2.96 * 10^-19 J.
6. Finally, find the energy of 0.50 moles of photons: Energy = (0.50 moles) * (6.022 * 10^23 photons/mole) * (2.96 * 10^-19 J/photon) = 8.92 * 10^4 J.
So, the energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.92 * 10^4 Joules.
Learn more about photons: Which of the following could be the energy of a photon in the visible range? https://brainly.com/question/15946945
#SPJ11
The energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.93 x \(10^4\) J.
To find the energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm, we can use the following steps:
1. Convert the wavelength to meters: 671 nm = 671 x \(10^{(-9)}\) m
2. Calculate the energy of a single photon using Planck's equation: E = h * c / λ, where E is the energy, h is the Planck's constant (6.626 x \(10^{(-34)}\) Js), c is the speed of light (3.0 x \(10^8\) m/s), and λ is the wavelength in meters.
3. Calculate the total energy of 0.50 moles of photons by multiplying the energy of a single photon by Avogadro's number (6.022 x \(10^{(23)}\) particles/mole) and the number of moles (0.50).
Step-by-step calculation:
1. λ = 671 nm = 671 x \(10^{(-9)}\) m
2. E (single photon) = (6.626 x \(10^{(-34)}\) Js) * (3.0 x \(10^8\) m/s) / (671 x \(10^{(-9)}\) m) = 2.967 x \(10^{(-19)}\) J
3. Total energy = E (single photon) * 0.50 moles * (6.022 x \(10^{(23)}\) particles/mole) = (2.967 x \(10^{(-19)}\) J) * 0.50 * (6.022 x \(10^{(23)}\)) = 8.93 x \(10^4\) J
So, the energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.93 x 10^4\(10^4\) J.
To learn more about moles, refer:-
https://brainly.com/question/26416088
#SPJ11
It is believed the major reason for the decline in amphibian species is _______.
a.
death through disease
b.
changing reproductive habits
c.
mutation
d.
climate change
Answers
Answer: It is believed the major reason for the decline in amphibian species is death through disease.
Explanation:
The major reason for the decline in amphibian species is climate change.
Amphibians are organisms that can live on land and in water. They are
usually found around moist areas because they need water bodies to cool
off in order for their survival.
Climate change is characterized by an increase in temperature which
doesn't favor them as they lose more water which thereby threatens survival
and leads to their decline in the ecosystem.
Read more on https://brainly.com/question/17825266
he glucose meter measures the current produced during Reaction 2. If 0.67 μmol of electrons were measured, what mass of glucose was present in the sample? (Note: The molar mass of glucose is 180 g/mol = 180 μg/μmol.)
Answers
We need to understand the reaction that the glucose meter is measuring. Reaction 2 likely involves the oxidation of glucose to produce electrons, which are then measured by the glucose meter.
We are given that 0.67 μmol of electrons were measured. To find the mass of glucose present in the sample, we can use stoichiometry to relate the number of electrons produced to the amount of glucose consumed.
The balanced reaction for the oxidation of glucose is:
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
We can see that for every glucose molecule oxidized, 6 electrons are produced. Therefore, the number of moles of glucose present in the sample is:
0.67 μmol / 6 = 0.112 μmol
To convert this to mass, we can use the molar mass of glucose:
0.112 μmol x 180 μg/μmol = 20.16 μg
Therefore, there were approximately 20.16 μg of glucose present in the sample.
to learn more about glucose
https://brainly.com/question/8880586
#SPJ11
is the average mass of one formula unit, measured in unified mass units (u). What is the formula mass of Cu2O?
Answers
The formula mass of Cu2O is approximately 143.09 u.
How do you calculate the formula mass of a compound?The formula mass of a compound is calculated by summing the atomic masses of all the atoms in its chemical formula.
What is the difference between molecular mass and formula mass?Molecular mass is the sum of the atomic masses of all the atoms in a molecule, while formula mass is the sum of the atomic masses of all the atoms in a formula unit of an ionic compound.
The formula mass of Cu2O is calculated by adding the atomic masses of two copper atoms and one oxygen atom, which gives:
Formula mass of Cu2O = (2 x atomic mass of Cu) + (1 x atomic mass of O)
= (2 x 63.55 u) + (1 x 15.99 u)
= 127.10 u + 15.99 u
= 143.09 u
Learn more about Molecular mass here:
https://brainly.com/question/14122402
#SPJ1
If neon gas travels at 449 m/s at a given temperature, estimate the rate of diffusion of butane gas, C4H10, at the same temperature.
Answers
The rate of diffusion of butane gas at the same temperature is approximately 240 m/s.
Rate of diffusion of a gasThe rate of diffusion of a gas is directly proportional to its velocity at a given temperature. Therefore, we can use the ratio of the velocities of neon and butane gases to estimate the rate of diffusion of butane gas.
The molar mass of neon is 20.18 g/mol, and the molar mass of butane is 58.12 g/mol. Since both gases are at the same temperature, their velocities are proportional to the square root of the ratio of their molar masses:
\(\sqrt{(20.18 g/mol / 58.12 g/mol) }\)≈ 0.534
Therefore, we can estimate that the rate of diffusion of butane gas is about 0.534 times the velocity of neon gas. Multiplying the velocity of neon gas by this ratio, we get:
449 m/s x 0.534 ≈ 240 m/s
So, we can estimate that the rate of diffusion of butane gas at the same temperature is approximately 240 m/s.
Learn more on rate of diffusion here:https://brainly.com/question/30622904
#SPJ1