Answers
Answer: Water as its the universal solvent
Related Questions
Suggest reasons for and against the inclusion of hydrogen in the main group of the periodic table
Answers
Answer:
See explanation
Explanation:
Hydrogen has only one valence electron. Sometimes, hydrogen is included as part of group 1 or 17 in the periodic table. Is this correct? Well, the answer to this question is not so straight forward!
Let us remember that hydrogen forms a univalent positive ion H^+ just like the group 1 elements, but it also forms a univalent negative ion H^- just like group 17 elements. Also, hydrogen is a gas and forms a molecular diatomic compound just like group 17 elements. This is not the case with other group 1 elements. We can see that hydrogen is chemically and physically dissimilar to other group 1 elements hence one can logically argue against its inclusion in group 1. Also, it is not a halogen, so we can also argue against its inclusion in group 17.
However, it is convenient to include hydrogen in the main groups 1 or 17 when discussing its chemical properties in order to make it easier to assimilate. If we place hydrogen in group 1, we can understand that it forms univalent positive ions. Similarly, if we place it in group 17, we understand that it forms univalent negative ions.
Most times hydrogen is just written separately at the top right corner of the periodic table and not as a member of any group because of the issues outlined above.
Hydrogen is placed in the main group of the periodic table since it has only one electron in it and it also behaves like alkali metal properties.
Hydrogen atom:Hydrogen forms a univalent positive ion \(H^+\)group 1 elements, but it also forms a univalent negative ion \(H^-\)group 17 elements. Also, hydrogen is a gas and forms a molecular diatomic compound just like group 17 elements.
This is not the case with other group 1 elements. We can see that hydrogen is chemically and physically dissimilar to other group 1 elements hence one can logically argue against its inclusion in group 1. Also, it is not a halogen, so we can also argue against its inclusion in group 17.
If we place hydrogen in group 1, we can understand that it forms univalent positive ions and if it is placed in halogen group it will form univalent negative ion.
Find more information about Hydrogen here:
brainly.com/question/25597694
How to water a plant
Answers
Pour water and put near sunlight :)
What is the second law of thermodynamics, in terms of heat/energy
Answers
Answer:
Second law of thermodynamics states that as energy is transferred or transformed from one form to another, more and more of it is wasted.
Explanation:
Second law of thermodynamics states that as energy is transferred or transformed from one form to another, more and more of it is wasted.
Second law of thermodynamics explains that it is impossible to convert an energy to another form of energy with 100% efficiency.
Example the conversion of heat energy to mechanical energy cannot be 100 % efficient. After the process of heating a gas to increase its pressure to drive a piston, there is always some leftover heat in the gas that cannot be used to do any additional work, this leftover heat is known as waste heat.
Can some one help me plz

Answers
Answer:
what does it say its blurry
Explanation:
write the balanced chemical equations for the neutralization reactions between the listed acid and base. (remember, acids and bases react to form water and a type of salt): a) hi and naoh b) h2co3 and sr(oh)2 c) ca(oh)2 and h3po4
Answers
The balanced chemical equations are :
a) HI + NaOH ----> NaI + H₂O
b) H₂CO₃ + Sr(OH)₂ ----> SrCO₃ + 2H₂O
c) 3Ca(OH)₂ + 2H₃PO₄ ----> Ca(PO₄)₂ + 6H₂O
The acid base neutralization reaction is reaction when an acid and a base will react to form the water and a salt and it involves the combination of the H⁺ ions and OH⁻ ions to produce water. The neutralization of the strong acid and the strong base has a pH equal to 7.
The balanced equation of the neutralization reactions are :
HI + NaOH ----> NaI + H₂O H₂CO₃ + Sr(OH)₂ ----> SrCO₃ + 2H₂O 3Ca(OH)₂ + 2H₃PO₄ ----> Ca(PO₄)₂ + 6H₂OTo learn more about balanced equation here
https://brainly.com/question/19340604
#SPJ4
As the temperature of a material increases, the average __(blank)__ of its particles increases.
kinetic energy
mass
specific heat
potential energy
Answers
Kinetic energy
As the temperature of a material increases, the average kinetic energy of its particles also increases. This is because the kinetic energy of a particle is directly proportional to the temperature of the system. Kinetic energy is the energy that an object possesses due to its motion. It is given by the formula: KE = (1/2) * m * v^2, where KE is the kinetic energy, m is the mass of the object, and v is the velocity of the object.
As the temperature of a material increases, the particles that make up the material begin to move faster and with more energy. This increase in the movement and energy of the particles results in an increase in their average kinetic energy.
Learn more about Kinetic energy here:
https://brainly.com/question/20658056
As we will soon see in Section 4.5, gasoline is a complex mixture of alkanes and other hydrocarbons, and the term "octane rating" is used as a standard measure of the performance of engine fuel. In the petroleum industry, one area of interest is the use of catalysts to convert unbranched alkanes and branched alkanes with higher octane ratings (Journal of the Japan Petroleum Institute 2004, 47, 1–10). For example, heptane can undergo isomerization in the presence of suitable catalysts to give eight different constitutional isomers, all of which are branched alkanes with the molecular formula C7H16. Draw all eight constitutional isomers, making sure not to draw the same compound twice.
Answers
Answer:
Explanation:
C₇H₁₆ is heptane and it has nine isomers (n-heptane and the remaining 8 constitutional isomers). Constitutional isomers are compounds with the same molecular formula but different structural formula or atom connectivity.
The constitutional isomers of n-pentane are 2-methylhexane, 3-methylhexane, 2,2-dimethylpentane, 2,3-dimethylpentane, 2,4-dimethylpentane, 3,3-dimethylpentane, 3-ethylpentane and 2,2,3-trimethylbutane. There structures are in the picture attached

3. When a substance is dissolved in water,___.
it lowers the boiling point.
the is prevented from boiling.
it raises the boiling point.
it raises the freezing point.
Answers
Answer:
it raises the boiling point.
Explanation:
BONUS:When a substance is dissolved in water,the boiling point will INCREASEand the freezing point will DECREASE.One way to limit deforestation is by
A. Practicing crop rotation
b. Increaseing soil fertilization
C. Increase of wood products
D. Using trees grown on tree farms
Thank you!
Answers
Answer:
the answer should be d I'm pretty sure.
Comparing Designs
Question 1
The students worked on their clay boats. The only building material allowed was the clay. Finally they teams had the first trial run and Boat 1 and Boat 2 were in hot competition. Boat 1 had a V-shaped hull while Boats 2 and 3 had wide, U-shaped hulls. Based on the results of the first trial run, what suggestion(s) would you give to Team 3?
Responses
A Make the sides lower and the base of the boat larger.Make the sides lower and the base of the boat larger.
B Rework the hull making sure there are no leaks.Rework the hull making sure there are no leaks.
C Try a V-shaped hull like Team 1.Try a V-shaped hull like Team 1.
D Cover the exterior with duct tape.Cover the exterior with duct tape.
Question 2
As we said, Boat 1 and Boat 2 were in hot competition. Boat 1 had a V- shaped hull with high sides to prevent water from entering the boat. Boat 2 had a U-shaped hull with a flat bottom and lower sides. If Boat 1 wants to beat Boat 2 in the second trial, how should the team modify the design?
Responses
A Line the inside of the boat with waterproof material.Line the inside of the boat with waterproof material.
B Make the sides higher to hold more cargo.Make the sides higher to hold more cargo.
C Leave the hull alone; be slow and careful when adding cargo.Leave the hull alone; be slow and careful when adding cargo.
D Lower the sides; make the base larger.
Answers
The research setup is a comparative analysis of several boats in an effort to understand their performance on the water and the differences between them because little would be learned from studying just one boat. Seven boats have been chosen for additional examination. They are all either "traditional" boats or boats that have been found in archaeology. Smaller open boats are the main focus.
The mass of an object per unit volume is what is meant by density. A brick, for instance, weighs more than a Styrofoam block of equal size (volume), indicating that the brick is denser than the Styrofoam. An object will float in a liquid if it has a lower density than the liquid. An object will sink in a liquid if it has a higher density than the liquid.
Instruct students to work in teams of two. First, they design their boats, keeping in mind that the boats must float while holding weight and that they have a limited amount of clay to use. Students can test their designs and make changes as often as they like. The goal is to create boats that can hold as much weight as possible while staying afloat.
Learn more about mass from here;
https://brainly.com/question/30388313
#SPJ1
1. Determine the volume of SO2 (at STP) formed from the reaction of 96.7 mol FeS2 and 55.0 L of O2 at 358 K and 1.20 atm.
4 FeS2(s) + 11O2(g) 2Fe2O3(s) + 8SO2(g)
Answers
Answer:
40.0L of SO2 are produced
Explanation:
To solve this question we need to find the moles of O2 using PV = nRT in order to find the moles. Thus, we can find the limiting reactant and the moles (And volume) of SO2 produced as follows:
Moles O2:
n = PV/RT
n = 1.20atm*55.0L / 0.082atmL/molK*358K
n = 2.25 moles of O2.
Clearly, limiting reactant is O2.
The moles of SO2 produced are:
2.25 moles of O2 * (8mol SO2 / 11mol O2) = 1.6351 moles SO2
Volume SO2:
V = nRT/P
V = 1.6351 moles SO2*0.082atmL/molK*358K / 1.20atm
V = 40.0L of SO2 are produced
1. Marie left her briefcase at home and had to return to get it. At which point did she turn back around to go home?
Answers
Answer:
She turned back at the point at which she forgot she left her briefcase. If this isn't the correct answer, please be more specific in the question.
Explanation:
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
(6 pts) A compound was analyzed and found to contain 14.14% C, 2.38% H, and 83.48% Cl.
Determine the empirical formula for this compound.
Answers
The empirical formula for this compound is CH₂Cl₂.
C = 14.14% / 12 = 1.17
H = 2.38% / 1 = 2.38
Cl = 83.48% / 35.5 = 2.35
Now, divide by 1.17
C = 1.17 / 1.17 = 1
H = 2.38 / 1.17 = 2
Cl = 2.35 / 1.17 = 2
Therefore, The empirical formula for this compound is CH₂Cl₂.
In chemistry, the empirical formula of a chemical compound is the best entire variety ratio of atoms found in a compound.
There are three main types of chemical formulas: empirical, molecular and structural. Empirical formulas display the best whole-range ratio of atoms in a compound, molecular formulas show the quantity of every kind of atom in a molecule, and structural formulas display how the atoms in a molecule are bonded to every different.
Learn more about empirical formula here:- https://brainly.com/question/1603500
#SPJ9
how to name Type 2 ionic compounds. AuCl3
Answers
To name Type 2 ionic compounds such as AuCl₃, you need to use the Stock system or Roman numeral system to indicate the oxidation state of the cation. Some steps are; Identify the cation, Determine the charge, Write the name, and combine two names.
Here are the steps to name AuCl₃; Identify the cation and anion. In this case, the cation is Au³⁺ and the anion is Cl⁻.
Determine the charge on the cation by using the anion's charge and balancing the charges to zero. Since Cl⁻ has a charge of -1 and there are three Cl⁻ ions in the compound, the total negative charge is -3. Therefore, the Au³⁺ ion has a charge of +3.
Write the name of the cation first, followed by the name of the anion with an -ide ending. Since the cation is Au³⁺, we use the name "gold(III)" to indicate the oxidation state of +3. The anion is Cl⁻, so we add the -ide ending to get "chloride".
Combine the two names to get the compound's name: "gold(III) chloride".
Therefore, the name of the Type 2 ionic compound AuCl₃ is "gold(III) chloride".
To know more about ionic compounds here
https://brainly.com/question/9167977
#SPJ1
25.00 g of aluminum sulfide and 50.00 g of water react until the limiting reagent is used up: which is the limiting reagent? what is the max mass of hydrogen sulfide that can form?
Answers
The maximum mass of hydrogen sulfide that can form is 17.01 g.
What is Limiting Reagent?
A limiting reagent, also known as a limiting reactant, is the substance that is completely used up in a chemical reaction and limits the amount of product that can be formed. It is the reactant that is present in the smallest stoichiometric amount and, as a result, determines the theoretical yield of the reaction. The other reactants may be present in excess and may not be completely used up.
To determine which reactant is the limiting reagent, we need to calculate the amount of product that each reactant would produce and see which one produces less.
The balanced chemical equation for the reaction between aluminum sulfide and water is:
First, we need to calculate the amount of moles of each reactant:
moles of Al2S3 = mass ÷ molar mass = 25.00 g ÷ 150.16 g/mol = 0.1664 mol
moles of H2O = mass ÷ molar mass = 50.00 g ÷ 18.02 g/mol = 2.776 mol
Next, we need to determine the limiting reagent. From the balanced equation, we can see that one mole of Al2S3 reacts with 6 moles of H2O. Therefore, the amount of H2O required to react with 0.1664 mol of Al2S3 is:
0.1664 mol Al2S3 × 6 mol H2O/mol Al2S3 = 0.9984 mol H2O
Since we have 2.776 mol of H2O available, we have more than enough water to react with the 0.1664 mol of Al2S3. This means that aluminum sulfide is the limiting reagent.
To calculate the maximum mass of hydrogen sulfide that can form, we need to use the amount of moles of Al2S3 as the basis of our calculation. From the balanced equation, we know that 1 mole of Al2S3 produces 3 moles of H2S. Therefore:
moles of H2S = moles of Al2S3 × 3 mol H2S/mol Al2S3 = 0.1664 mol × 3 mol H2S/mol Al2S3 = 0.4992 mol H2S
Finally, we can calculate the mass of hydrogen sulfide produced using its molar mass:
mass of H2S = moles of H2S × molar mass = 0.4992 mol × 34.08 g/mol = 17.01 g
Therefore, the maximum mass of hydrogen sulfide that can form is 17.01 g.
Learn more about Limiting Reagent from given link
https://brainly.com/question/30628347
#SPJ1
i need help on this one

Answers
Answer:
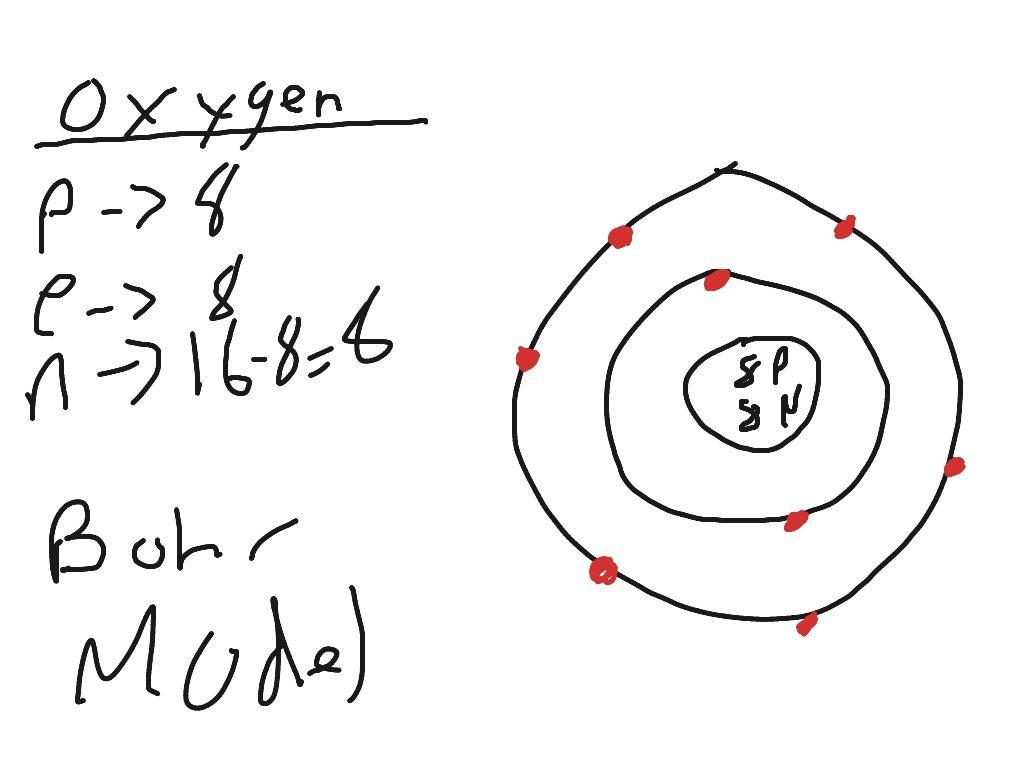
Ok so first it wants the atomic number is the atomic number would be 8.
Atomic mass is next which is 15.9
number of protons : 8 and the number of electrons is also 8
Now to find the number of neutrons ( have no charge) you have to subtract your atomic mass number and the total of protons and electrons so 15.999-16=0.001
Explanation:
I hope this helps this is 100% right I went over it many times.
Answer:
here
Explanation:
Which of the following is involved in bonding between atoms?
Select one:
O a. neutrons
O b. protons
O c. electrons
O d. the nucleus
Answers
The following is involved in bonding between atoms is electron
There are four type of chemical bonds essential for life to exist and it is ionic bond, covalent bond, hydrogen bond, and Vander wall interaction and all of these are different kind of bond to play various role in biochemical interaction and the electron on the outermost energy level of the atom are called valence electron and the valence electron are involved in bonding one atom yo another
Know more about atom
https://brainly.com/question/18086077
#SPJ9
Need answers asap!!!!!!!!!

Answers
The water cycle, also known as the hydrologic cycle, is the continuous movement of water on, above, and below the surface of the Earth.
What is the water cycle?The water cycle involves a series of physical processes, including evaporation, condensation, precipitation, and runoff, that work together to move water from one location to another and to maintain the balance of water on Earth.
The water cycle begins when water from oceans, lakes, rivers, and other bodies of water evaporates into the atmosphere due to the heat from the sun. As water vapor rises into the atmosphere, it cools and condenses into clouds. When the clouds become saturated with water vapor, precipitation occurs in the form of rain, snow, sleet, or hail.
Learn more about water cycle:https://brainly.com/question/31195929
#SPJ1
In Part C of this lab, you will measure the mass, height and diameter of four cylinders composed of some unknown material. Calculate the
volume (in cm³) of a cylinder with a measured height of 11.76 cm and a diameter of 7.22 cm. Type your answer in decimal notation and
include units
Answers
The cylinder's volume is roughly 479.85 cm3 at a height of 11.76 cm and a diameter of 7.22 cm.
Is the diameter of a cylinder measured with a vernier caliper?With no zero error, the diameter of a cylinder can be measured with vernier calipers. The Vernier scale's zero is discovered to be between 5.10 cm and 5.15 cm on the main scale. The 50 divisions on the Vernier scale are comparable to 2.45 cm.
V = πr²h
where r is the cylinder's radius, h is its height, and is the mathematical constant pi (approximately equal to 3.14159).
We must first determine the cylinder's radius in order to compute the volume of the cylinder with a height of 11.76 cm and a diameter of 7.22 cm:
r = d/2 = 7.22 cm / 2 = 3.61 cm
Now that the parameters have been entered, we can compute the cylinder's volume:
V = π(3.61 cm)²(11.76 cm)
V = 479.85 cm³
To know more about volume visit:-
https://brainly.com/question/25252629
#SPJ1
Metal ions are typically larger than their corresponding neutral atoms
Answers
Answer) Smaller
Physical methods of monitoring the rate of a chemical reaction
Answers
There are several physical methods that can be used to monitor the rate of a chemical reaction are; Spectrophotometry, Conductometry, and Turbidity measurement
Spectrophotometry involves measuring the changes in the intensity of light absorbed or transmitted by a solution during a chemical reaction. Spectrophotometers are used to measure the amount of light absorbed or transmitted by a sample at different wavelengths.
Conductometry involves measuring the changes in electrical conductivity of a solution during a chemical reaction. Conductivity meters are used to measure the electrical conductivity of a solution, which can change as the concentration of ions in the solution changes during a chemical reaction.
Turbidity measurement involves measuring the changes in the clarity or turbidity of a solution during a chemical reaction. Turbidimeters or nephelometers can be used to measure the amount of light scattered by a sample, which can change as particles form or dissolve during a reaction.
To know more about Spectrophotometry here
https://brainly.com/question/31440604
#SPJ1
--The given question is incomplete, the complete question is
"What are the physical methods of monitoring the rate of a chemical reaction?"--
With Regular/Diffuse Reflection, the __________ of the object will determine the SHARPNESS of reflection
Answers
Answer:
shape and texture.... .......
With regular / diffuse reflection, the angle and color of the object will determine the sharpness of reflection.
What is reflection ?When two distinct media come together at an interface, a wave front might reverse direction so that it returns to the first medium, which is known as reflection. The reflection of light, sound, and water waves are typical examples.
Reflection offers the brain a chance to stop in the middle of the chaos, organize observations and experiences, take into account many interpretations, and come up with meaning. Learning from this meaning can then guide future behaviors and mindsets.
An image is a representation of an item created by light that has been reflected or refracted. A surface must be exceptionally smooth in order for reflection to provide an image that is both clear and crisp. An picture may not develop if the surface is even slightly uneven or lumpy.
Thus, with regular / diffuse reflection, the angle and color of the object will determine the sharpness of reflection.
To learn more about reflection follow the link;
https://brainly.com/question/15487308
#SPJ2
The numbers represent the change in energy for a chemical reaction. They are calculated by subtracting the energy of products from the energy of reactants. Which number would result from an endothermic reaction?
Answers
Answer:
800
Explanation:
took the test and got it right
The numbers represent the change in energy for a chemical reaction. They are calculated by subtracting the energy of products from the energy of reactants. 800 would result from an endothermic reaction.Therefore, the correct option is D.
What are the various types of reactions?
There are different types of reactions, such as the endothermic reaction, where the energy is used for the product's formation, and the exothermic reaction.
The energy is released in an exothermic reaction, resulting in a negative result, whereas the energy is used in an endothermic reaction, resulting in a positive result.
Any chemical reaction that absorbs heat from its surroundings is an endothermic reaction. The absorbed energy serves as the activation energy for the reaction to take place.
Thus, 800 would result from an endothermic reaction, the correct option is D.
Learn more about the types of reactions here.
brainly.com/question/23184814
#SPJ2
Your question is incomplete, most probably your question was
The numbers represent the change in energy for a chemical reaction. They are calculated by subtracting the energy of products from the energy of reactants. Which number would result from an endothermic reaction?
–212
0
-1
800
Which of the following is NOT true of zinc?
-Excess zinc can decrease copper absorption.
-Grains are the most reliable food sources of zinc.
-All of its functions involve it acting as a cofactor for enzymes.
-It binds to most proteins in the body.
Answers
while zinc is an important mineral with numerous functions in the body, it is not true that grains are the most reliable food source of zinc. A balanced diet that includes a variety of foods can provide adequate zinc intake for most people.
How to solve the problem?
The statement that is NOT true of zinc is "Grains are the most reliable food sources of zinc." While grains can be a source of zinc, they are not necessarily the most reliable source.
Zinc is an essential mineral that plays important roles in many biological processes, including immune function, protein synthesis, wound healing, and DNA synthesis. It is involved in various enzymatic reactions, and acts as a cofactor for many enzymes. Zinc is also important for proper growth and development, especially during childhood and adolescence.
Excess zinc intake can lead to decreased copper absorption, as both minerals compete for absorption in the intestines. This can lead to copper deficiency, which can cause anemia neutropenia, and other health problems. Therefore, it is important to maintain a balance between zinc and copper intake.
While grains can be a source of zinc, other foods such as meat, seafood, and dairy products are also good sources. Vegetarians and vegans may need to pay particular attention to their zinc intake, as plant-based sources of zinc may be less bioavailable than -based sources. Zinc supplements can also be used to prevent or treat deficiencies, but should be used with caution as excessive intake can have negative health effects.
In summary, while zinc is an important mineral with numerous functions in the body, it is not true that grains are the most reliable food source of zinc. A balanced diet that includes a variety of foods can provide adequate zinc intake for most people.
To know more about enzymes visit :-
https://brainly.com/question/14577353
#SPJ1
PLS HELP
Explain the theory of plate tectonics and how they have changed Earth's surface over time. Include the role of plate tectonics in the creation of landforms.
Answers
Answer:
The surface of earth sits on plates that move over time because of the pressure build up underneath.When the pressure releases the pressure goes through the cracks of the plates (Or Earth) pushing them apart causing earthquakes and volcano eruptions.
Explanation: Science
Using standard heats of formation, calculate the standard enthalpy change for the following reaction.
H2(g) + C2H4(g)--------C2H6(g)
Answers
Answer:
\(\Delta _rH=-136.27kJ/mol\)
Explanation:
Hello,
In this case, for the given reaction, the enthalpy of reaction is computed in terms of the enthalpies of formation as:
\(\Delta _rH=\Delta _fH_{C_2H_6}-\Delta _fH_{C_2H_4}-\Delta _fH_{H_2}\)
Whereas hydrogen, ethene and ethane enthalpies of formation are 0 kJ/mol, 52.47 kJ/mol and -83.8kJ/mol respectively. Therefore, we compute:
\(\Delta _rH=-83.8kJ/mol-52.47kJ/mol-0kJ/mol\\\\\Delta _rH=-136.27kJ/mol\)
Best regards.
The pressure of 134 mL of a sample of carbon dioxide is increased from 485 mm Hg to 1,222 mm Hg. Calculate the new volume of the carbon dioxide.
Answers
The new volume of a gas when the pressure is increased is 53.2 mL.
What is the new volume of a gas when the pressure is increased?The new volume of a gas when the pressure is increased is determined using the formula from Boyle's law given below:
P₁V₁ = P₂V₂
where;
P₁ is the initial pressure
V₁ is the initial volume
P₂ is the new pressure
V₂ is the new volume
Solving for V₂:
V₂ = P₁V₁/P₂
V₂ = 134 * 485 / 1222
V₂ = 53.2 mL
Learn more about Boyles's law at: https://brainly.com/question/1696010
#SPJ1
name the following ester ch3c=ooch3

Answers
Answer:
The answer is D in acellus
Explanation:
The total pressure of gas collected over water is 770.0 mmHg and the temperature is 23.0 degrees Celsius what is the pressure of hydrogen gas formed in mmHg?
Answers
Given that the total pressure of gas collected over water is 770.0 mmHg, we can assume that this gas mixture contains water vapor and hydrogen gas. We need to determine the partial pressure of hydrogen gas in the mixture.
First, we need to determine the vapor pressure of water at the given temperature of 23.0 degrees Celsius. According to a vapor pressure table, the vapor pressure of water at 23.0 degrees Celsius is 21.1 mmHg.
Next, we can use Dalton's Law of Partial Pressures to calculate the partial pressure of hydrogen gas:
Total pressure = Partial pressure of hydrogen gas + Partial pressure of water vapor
770.0 mmHg = Partial pressure of hydrogen gas + 21.1 mmHg
Partial pressure of hydrogen gas = 770.0 mmHg - 21.1 mmHg = 748.9 mmHg
Therefore, the pressure of hydrogen gas formed in the mixture is 748.9 mmHg.