Line M passes through the points (-5, 9) and (-1,9).
12
10-
8
6
2
-12-10-8-6-4-22
2
6 8 10 12 X
6
-10
-12-
Which is true of line M?
Answers
Answer:
line m is vertical line with an undefined slopel line correct answer is 6 8 10 12 x
Related Questions
Identify the number of electrons
Atomic mass: 24
Atomic number: 12
-2 charge
Answers
Answer:
24 is the answer of this question
Protons and electrons are equal if element has not become an ion. 12 protons +, and 12 negative electrons= atomic number of 12
Charge of -2 means there are:
/////. 14 negative electrons
calculate the mass percent water in mgso4 • 2 h2o
Answers
23.01% is the mass percent water in MgSO\(_4\)• 2 H\(_2\)O. The term "mass percentage" describes the proportion.
What is mass percent?One approach to indicate the concentration of such a dissolved component in a solution is by mass percentage. The term "mass percentage" describes the proportion of a compound's mass to the overall mass of a solution.
Calculate the mass percent concentration, for instance, of a solution made by combining 6 g of sodium bicarbonate (NaHCO3) and 10 g of sodium chloride (NaCl) in 120 g of water.
mass percent = (mass of water/mass of MgSO\(_4\)• 2 H\(_2\)O)×100
mass percent =( 36/156.39)×100
=23.01%
Therefore, 23.01% is the mass percent water in MgSO\(_4\)• 2 H\(_2\)O.
To know more about mass percent, here:
https://brainly.com/question/14990953
#SPJ1
2.00 L of a gas is at 0.50 atm. What is its volume at standard pressure?
Answers
The volume of the gas at standard pressure is 1.00 L.
To find the volume of a gas at standard pressure, we can use the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
At standard pressure, the pressure is defined as 1 atm. Given that the initial pressure is 0.50 atm, and assuming the number of moles and temperature remain constant, we can set up a proportion to find the final volume:
(P₁)(V₁) = (P₂)(V₂)
Substituting the known values, we have:
(0.50 atm)(2.00 L) = (1 atm)(V₂)
Simplifying the equation, we get:
1.00 atm * L = V₂
It is important to note that the ideal gas law assumes that the gas behaves ideally, meaning it follows certain assumptions such as negligible molecular volume and intermolecular forces. In reality, these assumptions may not hold true, especially at high pressures or low temperatures.
For such more questions on pressure
https://brainly.com/question/857286
#SPJ8
Classify each property according to whether it is displayed by metals or by nonmetals
a. low melting point b. shiny dull c. poor conductor d. ductile e. malleable f. brittle g. good conductor h. high melting point
Answers
a. low melting point: Nonmetals. b. shiny: Metals. c. poor conductor: Nonmetals. d. ductile: Metals. e. malleable: Metals. f. brittle: Nonmetals. g. good conductor: Metals. h. high melting point: Metals
a. Low melting point is generally displayed by nonmetals. Metals tend to have high melting points.
b. Metals are typically shiny due to their ability to reflect light.
c. Nonmetals are generally poor conductors of heat and electricity.
d. Metals are ductile, meaning they can be drawn into thin wires without breaking.
e. Metals are malleable, meaning they can be hammered into thin sheets or shapes without shattering.
f. Nonmetals are usually brittle, meaning they are prone to breaking or shattering when subjected to stress.
g. Metals are good conductors of heat and electricity.
h. Metals typically have high melting points compared to nonmetals.
It's important to note that these are general trends and not absolute characteristics for all metals or nonmetals. Some exceptions or variations may exist within specific elements or compounds.
Learn more about properties of metals and nonmetals here: brainly.com/question/638020
#SPJ11
What is the speed of a car that travels 1000 m in 45 seconds?
Answers
Answer:
80,000km/h
Explanation:
Answer: 12.5 hope this is right.
Explanation:
Rank the bonds in each set in order of increasing bond length and increasing bond strength: (a) C≡N, C≡O, C≡C; (b) P-I, P-F, P-Br. And Rank the bonds in each set in order of decreasing bond length and decreasing bond strength: (a) Si-F, Si-C, Si-O; (b) N=N, N-N, N≡N.
Answers
Explanation:
There is a well-known relationship between the bond length of a diatomic molecule and the atomic radius of its constituent atoms, known as the covalent radius. Specifically, the covalent radius of an atom is half the bond length between two identical atoms in a diatomic molecule.
Therefore, to determine the atomic radius of chlorine (Cl), we can use the bond length of fluorine (F2) and the fact that the two atoms in F2 are identical.
Since the bond length of F2 is given as 1.28 A, the covalent radius of fluorine is 1.28/2 = 0.64 A.
Since both fluorine and chlorine are halogens, they have similar electronic configurations and form similar covalent bonds. Therefore, we can use the covalent radius of fluorine as an estimate for the covalent radius of chlorine.
Thus, the atomic radius of chlorine is approximately 0.64 A.

Rank the bonds in each set in order of increasing bond length and increasing bond strength: (a) C≡N, C≡O, C≡C; (b) P-I, P-F, P-Br. And Rank the bonds in each set in order of decreasing bond length and decreasing bond strength: (a) Si-F, Si-C, Si-O; (b) N=N, N-N, N≡N
ChatGPT
(a) C≡C < C≡N < C≡O (increasing bond length); C≡O < C≡N < C≡C (increasing bond strength)
Explanation: In a series of molecules with the same central atom, the bond length decreases as the number of bonds between the central atom and the surrounding atoms increases. Therefore, in set (a), the C≡C bond is the shortest, followed by the C≡N bond, and then the C≡O bond. Similarly, the bond strength increases with the number of bonds between the central atom and the surrounding atoms. Therefore, the C≡C bond is the strongest, followed by the C≡N bond, and then the C≡O bond.
(b) P-F < P-Br < P-I (increasing bond length); P-I < P-Br < P-F (increasing bond strength)
Explanation: In a series of molecules with the same surrounding atom, the bond length increases as the central atom gets larger. Therefore, in set (b), the P-I bond is the longest, followed by the P-Br bond, and then the P-F bond. Similarly, the bond strength decreases as the central atom gets larger. Therefore, the P-I bond is the weakest, followed by the P-Br bond, and then the P-F bond.
(c) Si-O < Si-C < Si-F (decreasing bond length); Si-F < Si-C < Si-O (decreasing bond strength)
Explanation: In a series of molecules with the same central atom, the bond length increases as the electronegativity of the surrounding atom increases. Therefore, in set (c), the Si-F bond is the longest, followed by the Si-C bond, and then the Si-O bond. Similarly, the bond strength decreases as the electronegativity of the surrounding atom increases. Therefore, the Si-F bond is the weakest, followed by the Si-C bond, and then the Si-O bond.
(d) N≡N < N-N < N=N (decreasing bond length); N≡N > N-N > N=N (decreasing bond strength)
Explanation: In a series of molecules with the same central atom, the bond length decreases as the number of bonds between the central atom and the surrounding atoms increases. Therefore, in set (d), the N≡N bond is the shortest, followed by the N-N bond, and then the N=N bond. Similarly, the bond strength increases with the number of bonds between the central atom and the surrounding atoms. Therefore, the N≡N bond is the strongest, followed by the N-N bond, and then the N=N bond.
1.(a) In order of increasing bond length: C≡N, C≡C, C≡O and In order of increasing bond strength: C≡O, C≡C, C≡N and (b) In order of increasing bond length: P-F, P-Br, P-I and In order of increasing bond strength: P-I, P-Br, P-F. 2. (a) In order of decreasing bond length: Si-F, Si-O, Si-C and In order of decreasing bond strength: Si-O, Si-C, Si-F and (b) In order of decreasing bond length: N≡N, N=N, N-N and In order of decreasing bond strength: N≡N, N=N, N-N.
1. (a) This is because nitrogen is smaller than carbon, so the triple bond is shorter and stronger. Carbon-oxygen bonds are typically shorter and stronger than carbon-carbon bonds, so C≡O is shorter and stronger than C≡C. In order of increasing bond strength the order is P-I, P-Br, P-F because oxygen is more electronegative than carbon, so the carbon-oxygen bond is more polar and stronger.
(b) The bond length order is so because fluorine is smaller than bromine or iodine, so the bond is shorter and stronger. and the bond strength order is so because iodine is larger than fluorine or bromine, so the bond is weaker and longer.
2. (a) This is because fluorine is smaller than oxygen, so the bond is shorter and stronger. Oxygen is smaller than carbon, so the bond is shorter and stronger. In order of decreasing bond strength the order is Si-O, Si-C, Si-F because oxygen is more electronegative than carbon, so the carbon-oxygen bond is more polar and stronger. Fluorine is more electronegative than carbon, so the carbon-fluorine bond is more polar and stronger.
(b) The bond length order is so because the triple bond is shorter and stronger than the double bond, which is shorter and stronger than the single bond and the bond strength order is so because the triple bond is stronger than the double bond, which is stronger than the single bond.
To know more about bond strength, refer here:
https://brainly.com/question/30226871#
#SPJ11
certain atom has two energy levels whose energies differ by 2.5 eV. In order for a photon to excite an electron from the lower energy level to the upper energy level, what must be true about the energy of the photon
Answers
In order for a photon to excite an electron from the lower energy level to the upper energy level of a certain atom, the energy of the photon must be equal to the energy difference between the two energy levels, which in this case is 2.5 eV.
For a photon to excite an electron from the lower energy level to the upper energy level in an atom with two energy levels whose energies differ by 2.5 eV, the photon must have an energy equal to the difference between the two energy levels. In this case, the energy of the photon must be 2.5 eV.
This is because the photon's energy will be absorbed by the electron, allowing it to transition to the higher energy level.
to learn more about photon click here:
brainly.com/question/12015967
#SPJ11
Approximately how many joules of heat are required to raise the temperature of 20 grams of water from 30°C to 40°C?
A
134 J
B
168 )
C.
420 j
D.
840 J
Answers
The heat required : 840 J
Further explanationGiven
mass of water = 20 g
Temperature : 30°C to 40°C
Required
Heat to raise the temperature
Solution
Heat can be calculated using the formula:
Q = mc∆T
Q = heat, J
m = mass, g
c = specific heat, joules / g ° C (for water= 4.18 J/g° C)
∆T = temperature difference, ° C / K
The heat :
\(\tt Q=20\times 4.2\times (40-30)\\\\Q=\boxed{\bold{840~J}}\)
How many significant figures are in 0.0034 cm? a 2 b 3 c 1 d 4
Answers
Answer:
A. 2 sig figs
Explanation:
In the number 0.0034, the zeros are called leading zeros. The only function of leading zeros is to fix the decimal point. Leading zeros are not counted as significant figures.
Which variable will you
measure? How will you do this?
Answers
Measuring Variables
Performance measuresAttitudinal measuresBehavioral observationFactual InformationWeb-based data collection#SPJ1
Now select a longer period, say the fourth period, starting with the element in the 1A group. Again, go from left to right. What do you notice about the pattern of change in the electron configuration as you go from left to right?
Answers
Answer:
The pattern of change in the electron configuration increases from left to right across the period.
Explanation:
In the periodic table, elements having the same number of electrons in the outermost shell of their atoms are placed over one another in vertical columns. Each of the vertical columns is known as a group or family. while each of the resulting horizontal rows is known as a period or row.
There are 18 vertical columns and seven periods in the periodic table. Each period begins with an atom having a valence electron and ends with an atom having a complete outer shell structure of an inert gas.
The fourth period of the periodic table (d-block) consists of the transition elements.
The electron distribution into the energy levels of sublevels of atoms can be shown in any of the tree important electronic configuration.
Electronic configuration shows the sequence of filling electrons into the orbitals of the sublevels as guided by some principles.
The maximum number of electrons in the orbitals of sublevels are two for s-sublevel(one orbital) ; six for p-sublevel( three orbital); ten for d-sublevel ( five orbitals) and fourteen for f-sublevel( seven orbitals). This indicates that the maximum number of electrons in an orbital is two
In the Periodic table ,The sublevels with lower energies are filled up before those with higher energies. One important thing about this principle is that the sublevels do not fill up in numerical order. The pattern of filling is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f etc.
Answer:
In the electron configuration of potassium, I observe that after the 3p orbital is filled, the next electron enters the 4s orbital instead of the 3d orbital because 3d is at a higher energy than 4s. This pattern shows that orbitals fill up according to the Aufbau principle. As I move on right, the number of electrons increases by one for each element until vanadium is reached. The electron configuration of chromium shows all Half-filled orbitals in the 4s and 3d energy levels. Again, as I move right, an exception occurs at copper. Copper’s electron configuration shows completely filled 4s and 3d orbitals. This is an exception to the Aufbau principle to attain more stable electron configurations, whenever possible. Half-filled electron configurations are more stable than other configurations and completely filled electron configurations are the most stable.
A bottles contains 3.100 mL of a liquid. The mass of the liquid is 2.060 g. What is the density of the liquid? (BE SURE TO
SHOW ALL THREE PARTS: EQUATION, WORK, AND PROPER FULL ANSWER.
Answers
Answer:
0.66 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{2.06}{3.10} \\ = 0.664516..\)
We have the final answer as
0.66 g/mLHope this helps you
Which of these describes “Al”?
Answers
Answer:
The aluminium cation Al3+ which is small and highly charged; and is polarizing and bonds aluminium forms tend towards covalency.
Explanation:
Select the correct structure that corresponds to the name.
3,3-dimethylcyclopentene
Answers
Answer:
See image
Explanation:

Two samples of sodium chloride were decomposed into their constituent elements. One sample produced 2.84 g of sodium and 4.37 g of chlorine. Which of the following could be the results of the decomposition of the other sample, being consistent with the law of constant composition (also called the law of definite proportions or law of definite composition)?
a) 4.17 g of sodium and 3.75 g of chlorine
b) 4.17 g of sodium and 6.42 g of chlorine
c) 4.17 g of sodium and 1.05 g of chlorine
d) 4.17 g of sodium and 12.1 g of chlorine
Answers
Answer:
The correct answer is b) 4.17 g of sodium and 6.42 g of chlorine
Explanation:
According to the law of definite proportions a chemical compound is composed always by the same elements in the same proportions by mass. In this case, the proportion of the elements by mass will be 4.37 g of chlorine (Cl) per 2.84 g of sodium (Na):
4.37 Cl/2.84 g Na= 1.54
We can calculate the proportions of the results in order to see which is the correct:
a) 3.75 g Cl/4.17 g Na = 0.899
b) 6.42 g Cl/4.17 g Na = 1.539 ⇒ ≅1.54
c) 1.05 g Cl/4.17 g Na = 3.971
d) 12.1 g Cl/4.17 g Na = 2.901
The option in which the proportion Cl/Na is equal to 1.54 is option b
Both plant and animal cells contain:
Answers
structural, plant and animal cells are very similar because they are both eukaryotic cells. they both contain membrane-bound organelle such as the nucleus, endoplasmic, mitochondria etc
PLZ help, I'm really behind in school! I will mark brainliest for correct answers!
If the distance between two objects is reduced by a factor of 5, how will Fg be affected?
A. F g decreases to 1/25
B. F g increases by 5 times
C. F g decreases to 1/5
D. F g increases by 25 times
Answers
Answer:
Gravitational force is given by:
F=G×
d
2
m×M
Distance between two masses is increased s.t. new distance is D= 5 d
New gravitational force F
1
=F
Let on of the mass is changed to m
1
so as to maintain the same gravitational force.
Answer:
D
Explanation:
F g increases by 25 times
in the titration of acetic acid and naoh, the use of the color indicator mandates that excess base be present to detect the reaction endpoint. will this cause the calculated and reported molarity of acetic acid in vinegar to be slightly high or low?
Answers
In the titration of acetic acid and NaOH, the use of the color indicator mandates that excess base be present to detect the reaction endpoint. This cause the calculated and reported molarity of acetic acid in vinegar to be slightly higher
Titration is the process of the reaction between acids and bases in which a reaction is carried out under controlled conditions. One of the reactant titrants is added to an analyte. The concentration of the analyte is determined by the concentration of the titrant.
The equivalence point is the point at which equivalent quantities of reactants have been mixed. For an acid-base reaction, the equivalence point is where the moles of acid and the moles of the base would neutralize each other. At the equivalence point, the number of H⁺ ions is equal to the number s OH⁻ ions and is done visually with an indicator that changes the color at the equivalent point and it shows that titration has been accomplished, resulting in a slightly higher calculated molarity of acetic acid and a higher reported volume percent of acetic acid.
To read more about Titration visit:
brainly.com/question/28872480
#SPJ4
4. Samples of compound X, Y, and Z are analyzed, with results shown here.
Compound
X
Y
Z
Description
clear, colorless, liquid with strong odor
clear, colorless, liquid with strong odor
clear, colorless, liquid with strong odor
Mass of Carbon
1.776 g
1974 g
7.812 g
Mass of Hydrogen
0.148 g
0.329 g
0.651 g
Do these data provide example(s) of the law of definite proportions, the law of multiple proportions, neither, or both?
What do these data tell you about compounds X, Y, and Z?
Answers
The data can show us that X ,Y and Z are all esters
What is the law of definite proportions?The law of definite proportions, also known as Proust's Law, states that a given chemical compound always contains the same elements in the same proportion by mass.
This means that no matter how a compound is obtained or prepared, its elemental composition will always be the same. For example, water is always composed of hydrogen and oxygen in a ratio of 2:1, regardless of its source or method of preparation.
The law of definite proportions is a fundamental principle of chemistry and provides evidence for the atomic theory of matter.
Learn more about esters:https://brainly.com/question/10840252
#SPJ1
what do you think the term polyester fabric refers to
Answers
Polyester fabric refers to a type of synthetic textile material that is made from polyester fibers. Polyester is a polymer, which means it is made up of many repeating units of a single molecule. It is often blended with other fibers, such as cotton or rayon, to create fabrics that are durable, lightweight, and wrinkle-resistant.
Polyester fabric has a number of advantages over natural fibres, including resistance to stretching and shrinking, as well as resistance to wrinkles and creases. It is also relatively easy to care for, as it can usually be machine-washed and dried without any special treatment. Polyester fabric is commonly used in clothing, bedding, and home furnishings, as well as in industrial applications such as filter fabrics and insulation materials.
Learn more about Polyester fabric at https://brainly.com/question/16850410
#SPJ11
point(s) where only a gas is present b) temperature at which vaporization occurs c) point(s) where a mixture of a solid and a liquid are present d) point(s) where a gas turns into a liquid e) temperature at which the substance starts to freeze f) range where there is only a liquid
Answers
The solution for the above questions are gas phase, boiling point, the solid-liquid (melting point) phase, condensation point, freezing point and liquid phase respectively.
What is vaporization?Vaporization is the process by which a substance is transformed from its liquid or solid state into its gaseous (vapor) state. Boiling is the term for the vaporization process when conditions permit the formation of vapor bubbles within a liquid. Sublimation is the process of directly converting a solid into a vapor.
a) point(s) where only a gas is present: the gas phase
b) temperature at which vaporization occurs: the boiling point
c) point(s) where a mixture of a solid and a liquid are present: the solid-liquid phase boundary
d) point(s) where a gas turns into a liquid: the condensation point
e) temperature at which the substance starts to freeze: the freezing point
f) range where there is only a liquid: the liquid phase
To know more about vaporization, visit:
brainly.com/question/11651433
#SPJ4
______________Helps the earth continue to go around the sun
Gravity
Entropy
Magnetism
Inertia
Friction
Answers
A file ____ shows the location of a file or folder.A)matrixB)pathC)runD)circuit
Answers
The correct term to complete your question is "path." A file path shows the location of a file or folder.
File path literally denotes the location of a specific file.
In other words, this indicates that a file's path determines or specifies location of a file or a folder.
Consider a text file named abc that is located on the desktop. Its file location might be C:UsersHPDesktopabc.txt.
Other options are file type, which refers to the kind of file—it might be a text file, an image, etc.—and file extension, which is a suffix that tells what kind of file it is (like jpg, txt, or png).
To learn more about file path, visit:
https://brainly.com/question/19425103
#SPJ11
how are crystal structures affected with increasing atomic radius
Answers
Increasing atomic radius can have a significant impact on the crystal structure of a material, affecting parameters such as interatomic distances, coordination numbers, lattice parameters, symmetry, and mechanical properties.
As the atomic radius increases, there are several effects on the crystal structure of a material:
1. Interatomic Distance: With increasing atomic radius, the average distance between neighboring atoms in a crystal lattice also increases. This leads to a larger unit cell and a decrease in atomic packing density.
2. Coordination Number: The coordination number refers to the number of atoms surrounding an atom in a crystal lattice. As the atomic radius increases, the coordination number may change. For example, a smaller atom might have a higher coordination number, while a larger atom might have a lower coordination number. This can influence the overall structure and stability of the crystal.
3. Lattice Parameters: The lattice parameters, such as the lattice constant or unit cell dimensions, may be affected by changes in atomic radius. An increase in atomic radius can result in an expansion of the crystal lattice, leading to changes in the lattice parameters.
4. Symmetry: Changes in atomic radius can affect the symmetry of the crystal structure. Larger atoms may disrupt the symmetry and introduce distortions or vacancies in the crystal lattice.
5. Mechanical Properties: The mechanical properties of a material, such as its strength, hardness, and flexibility, can be influenced by changes in atomic radius. Alterations in atomic size can affect the bonding forces between atoms and, consequently, the material's mechanical behavior.
Learn more about atomic radius at https://brainly.com/question/13126562
#SPJ11
Could someone help me with this pls i need it asap chimestry
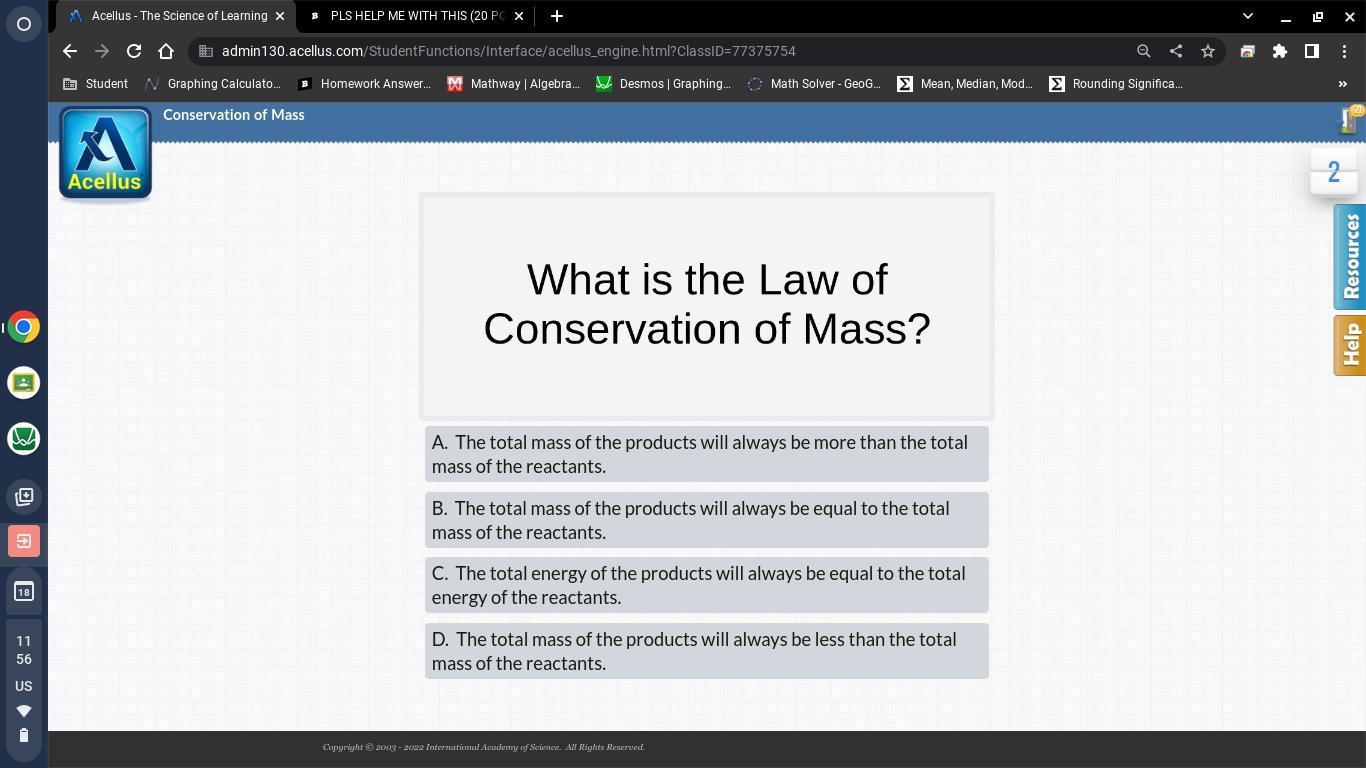
Answers
Answer:
b
Explanation:
the mass stays the same, it is only rearanged
Answer: B
Explanation: Law of Conservation of Mass states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so quantity can neither be added nor be removed.
CAN ANYONE TELL THE ANSWER

Answers
When water falls from great heights, its potential energy transforms to kinetic energy and this energy is converted electrical energy by power generators which produce a heat energy also. Thus, option b is correct.
What is energy transformation?One form of energy can be transformed to the other form naturally or mechanically. When a body is at rest, it possess potential energy. When it starts to move potential energy convert to kinetic energy.
When water falls from great heights it acquires more kinetic energy and it can be converted to electrical energy using turbines where some energy is lost as heat energy.
Therefore, the heat energy produced by the mechanical work of the turbine make the water heated. Hence, option b is correct.
To find more on energy transformation, refer here:
https://brainly.com/question/8210521
#SPJ1
how many grams of co2 would result from the complete combustion of 4.00 mol butane (c4h10)?
Answers
The complete combustion of 4.00 mol butane (C4H10) would result in 704.16 grams of CO2
To determine how many grams of CO2 would result from the complete combustion of 4.00 mol butane (C4H10), please follow these steps:
Step 1: Write the balanced chemical equation for the combustion of butane.
C4H10 + 6.5O2 -> 4CO2 + 5H2O
Step 2: Determine the mole ratio of butane to CO2.
From the balanced equation, we can see that for every 1 mole of butane, 4 moles of CO2 are produced. So, the mole ratio is 1:4.
Step 3: Calculate the moles of CO2 produced.
Since you have 4.00 moles of butane, use the mole ratio to find the moles of CO2:
4.00 moles of butane * (4 moles of CO2 / 1 mole of butane) = 16.00 moles of CO2
Step 4: Convert moles of CO2 to grams of CO2.
To do this, you need to know the molar mass of CO2, which is approximately 44.01 g/mol. Multiply the moles of CO2 by the molar mass:
16.00 moles of CO2 * 44.01 g/mol = 704.16 grams of CO2
Learn more about combustion : https://brainly.com/question/13251946
#SPJ11
lonic compounds can conduct electricity in
Answers
Answer:
Ionic compounds conduct electricity when molten (liquid) or in aqueous solution (dissolved in water), because their ions are free to move from place to place. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move
Explanation:
What do covalent bond and organic compound have in common?
Answers
Answer:
Covalent bonds are much more common in organic chemistry than ionic bonds. A covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons. The electrons located between the two nuclei are bonding electrons.
Explanation:
Atom Valence
Oxygen 2
Sulfur 2
Nitrogen 3
Carbon 4
p-methoxybenzaldehyde can be prepared from anisole using the gatterman-koch formylation. what mixture of reagents is necessary for this process?
Answers
A mixture of reagents is necessary, which includes Carbon monoxide (CO) ,Hydrochloric acid (HCl) and A Lewis acid catalyst .
The Gattermann-Koch formylation is a chemical reaction used to convert aromatic compounds, such as anisole, into aromatic aldehydes, like p-methoxy benzaldehyde. To perform this reaction :
1. Carbon monoxide (CO): It acts as a formylating agent, providing the necessary carbonyl (C=O) group to form the aldehyde product.
2. Hydrochloric acid (HCl): It serves as a catalyst, facilitating the reaction between anisole and the formylating agent. It also helps generate the necessary intermediate, formyl chloride, which reacts with anisole to form the desired product.
3. A Lewis acid catalyst: Commonly used catalysts are aluminum chloride \(AlCl_{3}\)or ferric chloride (\(FeCl_{3}\)). These catalysts activate the aromatic ring of anisole, making it more reactive towards electrophilic aromatic substitution.
Know more about Gattermann-Koch here :
brainly.com/question/27894163
#SPJ11