Is this correct?
If the temperature is below the melting point the substance is a solid
If the temperature is above the boiling point the substance is a gas
If the temperature is in between the boiling and melting points the substances a liquid.
Answers
If the normal melting point of a substance is below room temperature, the substance is a liquid at room temperature.
If both the normal melting point and the normal boiling point are above room temperature, the substance is a solid.
Related Questions
s 7.421 g of carbon, 0.779 g of hydrogen, 4.329 g of nitrogen, and 2.472 g of oxygen. the empirical formula of caffeine is
Answers
The empirical formula of a compound gives the simplest whole-number ratio of atoms present in the compound. To determine the empirical formula of caffeine, we need to calculate the moles of each element and then find the ratio between them. First, let's find the moles of each element by dividing their masses by their respective molar masses. The molar mass of carbon (C) is 12.01 g/mol, hydrogen (H) is 1.01 g/mol, nitrogen (N) is 14.01 g/mol, and oxygen (O) is 16.00 g/mol.
Moles of carbon (C):
7.421 g / 12.01 g/mol = 0.617 mol Moles of hydrogen (H): 0.779 g / 1.01 g/mol = 0.771 mol.Moles of nitrogen (N):
4.329 g / 14.01 g/mol = 0.309 mol Moles of oxygen (O): 2.472 g / 16.00 g/mol = 0.154 mol Next, we need to find the simplest whole-number ratio of these moles.To do this, we divide each mole value by the smallest mole value (0.154 mol in this case):
Moles of carbon (C) / 0.154 mol: 0.617 mol / 0.154 mol = 4 Moles of hydrogen (H) / 0.154 mol: 0.771 mol / 0.154 mol = 5 Moles of nitrogen (N) / 0.154 mol: 0.309 mol / 0.154 mol = 2 Moles of oxygen (O) / 0.154 mol: 0.154 mol / 0.154 mol = 1 The ratio of moles is approximately 4:5:2:1. Therefore, the empirical formula of caffeine is C4H5N2O. About CaffeineCaffeine, or more popularly caffeine, is a xanthine alkaloid compound in the form of crystals and tastes bitter which works as a psychoactive stimulant and mild diuretic. Caffeine was discovered by a German chemist, Friedrich Ferdinand Runge, in 1819. Caffeine can suppress appetite, so it can help control weight. In addition, caffeine can also stimulate thermogenesis, which is the process of converting food into heat and energy by the body. In addition, caffeine can also help improve performance while exercising. Caffeine in coffee can stimulate the nerves and brain, making a person unable to sleep, causing disturbed night sleep (insomnia), feeling excessively refreshed, which over time can shorten sleep time and prevent the body from sleep well. This can cause sleep disturbances such as insomnia.
Learn More About Caffeine at https://brainly.com/question/26670237
#SPJ11
42. Proton and electuron.
Puroton
Electron.
Differences between proton and electron in two points

Answers
Answer:
Protons:
- positive
- aka cation
- in the nucleus along with the neutrons
Electrons:
- negative
- aka anion
- situated in the orbital shells/configuration levels (there are many names)
2Na(s)+Cl2(g)â2NaCl(s)
In the reaction of sodium and chlorine, for every 2 moles of Na that react, _________ mol of electrons are transferred.
Answers
In the reaction of sodium and chlorine, for every 2 moles of Na that react, 2 mol of electrons are transferred. This is because sodium has one valence electron while chlorine has seven valence electrons.
Sodium has a tendency to lose this one electron and form a positively charged ion, while chlorine has a tendency to gain one electron and form a negatively charged ion. In this reaction, two sodium atoms lose their valence electrons and become two Na+ ions, while one chlorine molecule gains two electrons and becomes two Cl- ions.
The transfer of electrons is the basis of chemical reactions, and it is the way atoms try to achieve a stable electronic configuration by gaining or losing electrons. When two atoms have different tendencies to gain or lose electrons, they can form an ionic bond, which is the case in the reaction between sodium and chlorine. The resulting compound, sodium chloride, is held together by the electrostatic attraction between the Na+ and Cl- ions.
Overall, the reaction between sodium and chlorine is an example of a redox reaction, where oxidation and reduction occur simultaneously. Sodium is oxidized because it loses its valence electron, while chlorine is reduced because it gains an electron. This transfer of electrons leads to the formation of a new substance, sodium chloride.
To learn more about sodium, refer:-
https://brainly.com/question/29327783
#SPJ11
1. A potassium atom has a larger atomic radius than a sodium atom. What statement about potassium correctly explains this difference?
A) It has a larger nuclear charge
B) It has a lower electronegativity
C) It has more energy levels occupied by electrons
D) It has a lower ionization energy
2. Which of the following elements has the greatest electron affinity (largest negative value)?
A) Mg
B) Al
C) Si
D) P
E) S
3. The electron affinity of fluorine is essentially equal to
A) The negative of the ionization energy F
B) The ionization energy F-
C) The negative of the ionization energy F-
D) The ionization energy Ne
E) The negative of the ionization energy Ne
Answers
The electron affinity is the energy required to remove an electron from an atom to yield a negative ion.
We know that atomic size increases down the group as more shells are added. This is because, inter-electronic repulsion pushes the electrons in the outermost shell farther away from the nucleus. Hence potassium atom has a larger atomic radius than a sodium atom because It has more energy levels occupied by electrons.
Electron affinity increases across the period. The more nonmetallic an element is, the more negative its electron affinity. Hence, sulfur has the greatest electron affinity.
Electron affinity is the opposite of ionization energy. It therefore follows that, the electron affinity of fluorine is essentially equal to the negative of the ionization energy F.
Learn more: https://brainly.com/question/17696329
For the following reaction:
N₂(g) + 3H₂(g) → 2NH₃(g)
Identify the compositions which will produce same amount of NH₃
(a) 140 gm N₂ & 35 g H₂
(b) 18 g H₂ & 52 g N₂
(c) Total 20 moles of mixture having N₂ and H₂ present in stoichiometric ratio (No limiting reagent)
(d) 136 gm of mixture having mass fraction of H₂ = 6/34
Answer is option (a) and option (c), can someone please explain verifying ALL the options? Will mark you as the brainliest!
Answers
Okay, let's go through each option step-by-step:
(a) 140 gm N2 & 35 g H2
since the stoichiometry is 2NH3 : 3H2 : N2, for every 2 moles of NH3 produced, 3 moles of H2 and 1 mole of N2 react.
So, 140 gm N2 = 10 moles N2
35 gm H2 = 3 moles H2
Together they can produce 10/2 = 5 moles NH3. So this option produces the same amount of NH3.
(b) 18 g H2 & 52 g N2
H2 has 3 moles per 35 g so 18 g H2 = 2 moles H2
52 g N2 = 4 moles N2
Producing 2 * (2/3) = 4/3 = 2 moles NH3. This is less than options a and c.
(c) Total 20 moles of mixture having N2 and H2 in stoichiometric ratio.
With 20 moles total and in stoichiometric ratio, the moles of each will produce 2 moles of NH3. So this option also produces the same.
(d) 136 gm of mixture having mass fraction of H2 = 6/34
* Total mass = 136 g
* Mass fraction of H2 = 6/34 = 0.18
* So mass of H2 = 0.18 * 136 = 24 g
* Mass of 24 g H2 = 2 moles H2
* Remaining mass = 136 - 24 = 112 g is N2
* 112 g N2 = 8 moles N2
* Together 2 moles H2 and 8 moles N2 can produce 2 * (2/3) = 4/3 = 2 moles NH3.
This is less, so this option does not produce the same amount.
In summary, options a and c satisfy the criteria of producing the same amount (i.e. 5 moles) of NH3.
Let me know if this helps explain the problem! I can provide more details if needed.
(a) 140 g N₂ & 35 g H₂:
Moles of N₂ = 140 g / 28 g/mol = 5 mol
Moles of H₂ = 35 g / 2 g/mol = 17.5 mol
Limiting reactant: N₂
Moles of NH₃ produced = 5 mol N₂ × (2 mol NH₃/1 mol N₂) = 10 mol NH₃
(b) 52 g N₂ & 18 g H₂:
Moles of N₂ = 52 g / 28 g/mol = 1.857 mol
Moles of H₂ = 18 g / 2 g/mol = 9 mol
Limiting reactant: N₂
Moles of NH₃ produced = 1.857 mol N₂ × (2 mol NH₃/1 mol N₂) = 3.714 mol NH₃
(c) Total 20 moles of mixture having N₂ and H₂ present in stoichiometric ratio (No limiting reagent) :
Moles of N₂ = 20 mol × (1 mol N₂/3 mol H₂) = 6.67 mol
Moles of H₂ = 20 mol × (3 mol H₂/3 mol H₂) = 20 mol
Limiting reactant: N₂
Moles of NH₃ produced = 6.67 mol N₂ × (2 mol NH₃/1 mol N₂) = 13.34 mol NH₃
(d) 136 gm of mixture having mass fraction of H₂ = 6/34:
Let the mass of N₂ be x, then the mass of H₂ will be (136 - x) g.
Mass fraction of H₂ = mass of H₂/total mass
6/34 = ((136 - x)/2) / 136
x = 34 g
Mass of N₂ = 136 - 34 = 102 g
Moles of N₂ = 102 g / 28 g/mol = 3.64 mol
Moles of H₂ = 34 g / 2 g/mol = 17 mol
Limiting reactant: N₂
Moles of NH₃ produced = 3.64 mol N₂ × (2 mol NH₃/1 mol N₂) = 7.28 mol NH₃
Option (a) will produce the same amount of NH₃ as option (c) because both options have the same number of moles of N₂ and H₂ in the stoichiometric ratio. They are not limiting reagents, and the amount of NH₃ produced will be based on the moles of N₂.
Hope this helped!
What are some ways carbon dioxide enters our atmosphere and subsequently the air we breathe
Answers
Answer:
when organisms decompose, or burning fossil fuels and pollution and such
Explanation:
you decide to test your pillbugs' preference for an acidic environment versus a nonacidic environment. on one side of the chamber you place filter paper moistened with water. what is appropriate to place on the other side to test this variable?
Answers
you decide to test your pillbugs' preference for an acidic environment versus a nonacidic environment. on one side of the chamber you place filter paper moistened with water. Dry filter paper is appropriate to place on the other side to test this variable.
ABOUT PILLBUGSArmadillidiidae (Pillbugs) is a family of woodlice, a terrestrial crustacean group in the order Isopoda. Unlike members of some other woodlice families, members of this family can roll into a ball, an ability they share with the outwardly similar but unrelated pill millipedes and other animals. This ability gives woodlice in this family their common names of pill bugs or roly polies.Other common names include slaters, potato bugs, and doodle bugs. Most species are native to the Mediterranean Basin, while a few species have wider European distributions. The best-known species, Armadillidium vulgare, was introduced to New England in the early 19th century and has become widespread throughout North America.
Learn more about pillbugs at
https://brainly.com/question/26410019.
#SPJ4
What volume of H2 is produced at 315 K and 1.25 atm when 3.50 grams of Zn reacts with excess HCl?
Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq)
Answers
The volume of hydrogen gas produced at 315 K and 1.25 atm when 3.50 grams of zinc reacts with excess HCl is 1.16 L.
The balanced chemical equation for the reaction between zinc and hydrochloric acid is:
\(Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq)\)
We need to find the volume of hydrogen gas produced at 315 K and 1.25 atm when 3.50 grams of zinc reacts with excess HCl.
First, we need to determine the number of moles of zinc used in the reaction. The molar mass of zinc is 65.38 g/mol, so:
n(Zn) = 3.50 g / 65.38 g/mol = 0.0536 mol
Since 1 mole of zinc reacts with 1 mole of hydrogen gas, the number of moles of hydrogen gas produced is also 0.0536 mol.
Using the ideal gas law, PV = nRT, we can calculate the volume of hydrogen gas produced. We are given the temperature (315 K) and the pressure (1.25 atm), and the gas constant R is 0.0821 L·atm/(mol·K).
Substituting the values into the equation, we get:
V = (nRT) / P
V = (0.0536 mol) x (0.0821 L·atm/(mol·K)) x (315 K) / (1.25 atm)
V = 1.16 L
Therefore, the volume of hydrogen gas produced at 315 K and 1.25 atm when 3.50 grams of zinc reacts with excess HCl is 1.16 L.
To learn more about hydrogen gas , here
https://brainly.com/question/14692538
#SPJ1
The power to conduct elections is what type of power
Answers
Answer:
Unlike delegated powers, they are not listed specifically, but are guaranteed by the Tenth Amendment: "The powers not delegated to the United States by the Constitution, not prohibited by it to the States, are reserved to the States respectively, or to the people." Some traditional reserved powers include regulating ...
Explanation:
guys can you help me to fill in the missing blanks
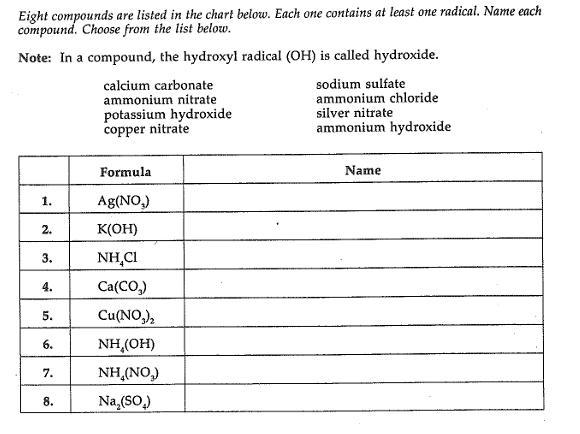
Answers
Answer:
Silver NitratePotassium HydroxideAmmonium ChlorideCalcium CarbonateCopper NitrateAmmonium HydroxideAmmonium NitrateSodium Sulfate25 ml of a 0. 10 m solution of magnesium chloride reacts with 25 ml of potassium hydroxide to form a magnesium hydroxide precipitate. What is the minimum concentration of potassium hydroxide necessary to completely precipitate all of the magnesium?.
Answers
Magnesium chloride, often known as MgCl2, can be produced chemically by extracting it from brine or seawater.
Magnesium chloride+ potassium hydroxide (25 ml )------>magnesium hydroxide
Magnesium chloride: what is it?
One magnesium (Mg) and two chloride ions make up magnesium chloride, also known as magnesium dichloride, magnesium (II) chloride, or chloromagnesite (Cl-).
Ionic halides, such as magnesium dichloride and related salts, have the appearance of fine, white to grey granules.
It has no smell and is very water soluble.It is frequently employed as medication for numerous cellular processes.Uses of MgCl2 (Magnesium Chloride)
Magnesium metals are produced using magnesium chloride as a precursor.utilised for soil stabilisation, dust management, and wind erosion.Fire extinguishers use this.used as an additive in food.utilised in the production of paper.is a component of disinfectants.a flocculating agent is used.To learn more about Magnesium hydroxide reaction, visit
https://brainly.com/question/15287665
#SPJ13
. If the volume, pressure, or amount of CO2 in solution was changed such that the amount of CO2 in solution decreased, what would happen to H2CO3 in the solution
Answers
If the amount of \(CO_{2}\) in solution decreases, the concentration of \(H_{2}CO_{3}\) in the solution would also decrease.
Dissolved \(CO_{2}\) concentration drops as solution \(CO_{2}\) decreases. \(CO_{2}\) combines with water to generate carbonic acid (\(H_{2}CO_{3}\)), therefore less \(CO_{2}\) means less carbonic acid in the solution.
The equilibrium reaction between dissolved \(CO_{2}\) and carbonic acid is:
\(CO_{2}\) (g) + \(H_{2}O\) (l) = \(H_{2}CO_{3}\) (aq)
Le Chatelier's principle states that an equilibrium system will change to mitigate stress. A reduction in \(CO_{2}\) in solution stresses equilibrium. To reduce stress, the equilibrium will shift left, lowering carbonic acid (\(H_{2}CO_{3}\)) concentration.
Thus, \(H_{2}CO_{3}\) concentration decreases with \(CO_{2}\) concentration.
Learn more about Le Chatelier's principle, here:
https://brainly.com/question/29009512
#SPJ4
If a dextrose solution had an osmolarity of 100 mosmol/l, what percentage (w/v) of dextrose (mw = 198.17) would be present?
Answers
The percentage (w/v) of dextrose in the solution is approximately 1.9817%. To determine the percentage (w/v) of dextrose in a solution with a given osmolarity, we need to calculate the amount of dextrose present in 100 mL of the solution.
First, we convert the osmolarity from mosmol/L to mosmol/100 mL:
100 mosmol/L = 100 mosmol/100 mL
Next, we calculate the number of moles of dextrose present in 100 mL of the solution:
Number of moles = Osmolarity (in mosmol/100 mL) / 1000
Number of moles of dextrose = 100 mosmol/100 mL / 1000 = 1 mosmol/100 mL
Now, we can calculate the mass of dextrose present in 100 mL of the solution:
Mass of dextrose = Number of moles of dextrose * Molecular weight of dextrose
Mass of dextrose = 1 mosmol/100 mL * 198.17 g/mol = 1.9817 g/100 mL
Finally, we can calculate the percentage (w/v) of dextrose:
Percentage (w/v) = (Mass of dextrose / Volume of solution) * 100
Percentage (w/v) = (1.9817 g/100 mL / 100 mL) * 100 = 1.9817%
Therefore, the percentage (w/v) of dextrose in the solution is approximately 1.9817%.
Learn more about osmolarity here:
https://brainly.com/question/32470302
#SPJ11
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
List at least three different properties that would help you determine whether the bars are made of the same metal.
Answers
Answer:
Refractive index
Heat capacity
Density
Explanation:
Properties that are used to identify materials are called intensive properties. These intensive properties are characteristic of the material and does not depend on the amount of material present.
Thus, if i want to identify whether the three bars are made of the same metal, i can look out for the following properties;
Refractive index
Heat capacity
Density
These properties are intensive properties thus they can be used to identify an unknown substance.
228 88ra 88228ra then decays through a series of beta-minus decays; eventually, another isotope of thorium, 228 90th 90228th , is formed. how many beta-minus decays will occur during this chain process?
Answers
2 beta-minus decays during this chain process.
In electron emission, also known as terrible beta decay, an unstable nucleus emits an energetic electron and an antineutrino with little or in all likelihood no relaxation mass, and a neutron in the nucleus will become a proton that stays inside the product nucleus.
Beta minus particle emission happens while the ratio of neutrons to protons inside the nucleus is simply too high. An extra neutron transforms into a proton and an electron. The proton remains in the nucleus and the electron is ejected energetically.
Beta-minus radiation, the emission of an electron and an anti-neutrino, occurs whilst a neutron transforms right into a proton. The opposite method, whereby a proton becomes a neutron thru the emission of a positron and a neutrino, is the supply of beta-high-quality radiation.
Learn more about beta-minus decays here:- https://brainly.com/question/15842303
#SPJ4
Calculate the pH of solutions prepared by a. Dissolving 4.8 g of lithium hydroxide in water to give 250 mL of solution
Answers
pH of the solution is 13.90.
What is pH ?The concentration of hydrogen (H+) ions in a solution, which is a measurement of acidity, is known as the pH of that solution. In pure water, hydrogen and hydroxyl (OH) ions are found in almost equal amounts.
A solution is acidic when there are too many hydrogen ions present, while a solution is basic when there are too few H+ ions or too many hydroxyl ions. The product of the concentrations of H+ and OH is the equilibrium constant for this reaction, Kw, which equals 10^14.
Given:Mass of lithium = 4.8 g
Volume of the solution = 250 mL
Formula used :[H3O+] = Kw/ [OH-]
Where,
[H3O+] : hydronium ion concentration
[OH-] : hydroxyl ion concentration
Kw : Equilibrium constant
Solution:moles of LiOH = 4.8/23.95 = 0.20 moles
strength of LiOH = 4 x 0.20 = 0.80 M
[H3O+] = Kw/ [OH-] = 10^(-14) / 0.80 = 1.25 x 10 ^(-14)
pH = - log [H3O+]
pH = - log ( 1.25 x 10 ^ (-14)) = 13.90
Therefor, pH is 13.90
To learn more about pH :
https://brainly.com/question/491373
#SPJ4
Complete this sentence. If mass remains the same while the volume of a substance ________, the density of the substance will_______________.
Answers
Answer:
If mass remains the same while the volume of a substance changes, the density of the substance will also change.
Answer:
BELOW
Explanation:
If mass remains the same while the volume of a substance decreases the density of the substance will increase.
substances that are made of particles and that move in a random motion I need this nowww!!!!
Answers
Answer:
ANY KENITIC PARTICLE
Particles in both liquids and gases collectively called fluids
Please mark as brainliest
Have a great day, be safe and healthy
Thank u
XD
3. If you had 152.5 g of CO and 24.5 g of H₂ gas, how many grams of CH₂OH could be produced?
CO+ _
CH₂OH
H₂ →→
H2
→
Answers
Find the number of moles in each reactant in the formula CO + 2 H2 CH3OH.
How to find the calculation?The reactant that yields the least amount of methanol is known as the limiting reagent. The limiting reagent is CO because it yields less CH3OH than other chemicals.1 mol CO divided by 28.01g CO to equal 152500 g CO results in 5444 mol CO.24500 g H2 x 1 mol H2 / 2.02 g H2 = 12129 mol H2In order to produce one mol of CH3OH, one mol of CO and two mol of H2 are required, according to the 1:2 mol ratio between CO and H2.However, there are only 5444 mol of CO, whereas 6064 mol of CH3OH can be produced by H2.1 mole of CH3OH divided by 5444 moles of CH3OH results in a number.= 174371 g = 174.4 kg.
To Learn more About number of moles refer To:
https://brainly.com/question/14357742
#SPJ1
The hindbrain includes the:
A. limbic system.
B. brain stem.
C. corpus callosum.
D. occipital lobes.

Answers
The hindbrain consists of the brain stem which encompasses the medulla oblongata, pons, and cerebellum. The Option B.
What structures are included in the hindbrain?These structures are located at the base of the brain and are responsible for essential functions such as regulating vital autonomic processes, controlling balance and coordination .
It also relays sensory and motor information between the brain and the rest of the body. The hindbrain plays a crucial role in maintaining basic bodily functions and facilitating smooth movement. Therefore, the Option B is correct.
Read more about hindbrain
brainly.com/question/17584815
#SPJ6
Which of the following compounds contains the greatest percent by mass of nitrogen

Answers
Answer:
\(A\colon NH_3\)
Explanation:
Here, we want to get which of the compounds have the highest percentage by mass of nitrogen
What we have to do here is to divide the mass of nitrogen atoms in the compound by the molecular mass of the compound
a) NH3
The atomic mass of nitrogen is 14 amu, the atomic mass of hydrogen is 1 amu
The molar mass is thus 14 + 3(1) = 17 g/mol
The percentage by mass of nitrogen will be
14/17 * 100 = 82.35 %
b) HCN
The atomic mass of carbon is 12 amu
Thus, we have the molar mass as
1 + 12 + 14 = 27 g/mol
Percentage of nitrogen by mass will be : 14/27 * 100 = 51.85 %
c) For N2O
The atomic mass of oxygen is 16 amu
The molar mass of the compound will be 14(2) + 16 = 44 g/mol
Percentage of nitrogen by mass will be = 28/44 * 100 = 63.64 %
d) NI3
The atomic mass of iodine is 127 g/mol
So the molar mass of the compound will be:
14 + 3(127) = 395 g/mol
Percentage by mass of nutrogen will be
14/395 * 100 = 3.54 %
From what we have, the highest percentage by mass of nitrogen is 82.35 % and that belongs to ammonia (NH3)
A cell membrane is more flexible than a brick wall. Why might many cells benefit from a flexible cell membrane?
Answers
A cell membrane is more flexible than a brick wall. Why might many cells benefit from a flexible cell membrane because it allows them to survive in and out of the cell.
The cell is surrounded by a membrane called as cell membrane . cell membrane is a semipermeable membrane. semipermeable membrane is the type of membrane which allows solvent particles to pass through it . or some molecules or ion . Therefore , many cells benefited by cell membrane as they can pass through it.
Thus, A cell membrane is more flexible than a brick wall. Why might many cells benefit from a flexible cell membrane because it allows them to survive in and out of the cell.
To learn more about cell membrane here
https://brainly.com/question/17568680
#SPJ1
How many grams Mn2O3 would be produced from the complete reaction of MnO2?
Zn + 2MnO2 + H2O --> Zn(OH)2 + Mn2O3
Mno2 : 86.94 g/mol
Mn2O3 : 157.88 g/mol [?] g Mn2O3
Answers
46.8 g if MnO2 would fully react to yield 84.5 g of Mn2O3. .According to stoichiometry, 46.8 g of manganese would undergo a full chemical reaction to yield 134.24 g of manganese(III) oxide.
Describe stoichiometry?The measurement of element or compound proportions in a biochemical reaction is known as stoichiometry.The laws of mass preservation and combining weights & volumes serve as the foundation for the linked relationships.
The reaction MnO2 Hydrochloric MnCl2 H2O Cl2 belongs to what kind?In this instance, Mno2 is reduction to MnCl2, and HCl is oxid to Cl2.b. An oxidation reaction is one in which a material either gains oxygen or loses hydrogen.
To know more about manganese visit:
https://brainly.com/question/29743410
#SPJ1
You bought 5 pounds, lbs of flour to bake bread. how many grams of flour did you buy?
Answers
If you bought 5 pounds, lbs of flour to bake bread, you bought: 2268 grams of flour
To solve this problem the we have to do the conversion of units with the given information.
Information about the problem:
mass(flour)=5 Lbsmass(flour) expressed in grams =?1 Lb = 453,6 gBy converting the mass units from (Lb) to (g) we have:
mass(flour)=5 Lbs * 453,6 g/ 1 Lb
mass(flour)=2268 g
What is unit conversion?It is the transformation of a value expressed in one unit of measurement into an equivalent value expressed in another unit of measurement of the same nature.
Learn more about unit conversion at: brainly.com/question/141163
#SPJ4

10. Explain the relationship you see for each chemical between the temperature and the
state of matter
11. Referencing your drawings, what happens to the space between particles as you go
from solid to liquid to gas?
12. Referencing your drawings, what happens to the speed of the particles as you go from
solid to liquid to gas?
Answers
Answer:
Explanation:
11. Referencing your drawings, what happens to the space between particles as you go
A 2.9 kg lump of aluminum is heated to 96oC and then dropped into 9.0 kg of water at 5.3oC. Assuming that the lump–water system is thermally isolated, what is the system's equilibrium temperature? Assume the specific heats of water and aluminum are 4186 and 900 J/kg-K, respectively.
Answers
To find the equilibrium temperature of the lump of aluminum and the water, we can use the principle of conservation of energy.
The heat lost by the aluminum lump is equal to the heat gained by the water when they reach thermal equilibrium.
The heat lost by the aluminum can be calculated using the formula:
Q_aluminum = m_aluminum * c_aluminum * (T_equilibrium - T_aluminum)
where:
m_aluminum is the mass of the aluminum lump (2.9 kg)
c_aluminum is the specific heat capacity of aluminum (900 J/kg-K)
T_equilibrium is the equilibrium temperature we want to find
T_aluminum is the initial temperature of the aluminum lump (96°C)
The heat gained by the water can be calculated using the formula:
Q_water = m_water * c_water * (T_equilibrium - T_water)
where:
m_water is the mass of the water (9.0 kg)
c_water is the specific heat capacity of water (4186 J/kg-K)
T_water is the initial temperature of the water (5.3°C)
Since the system is thermally isolated, the heat lost by the aluminum is equal to the heat gained by the water:
Q_aluminum = Q_water
Substituting the values into the equation:
m_aluminum * c_aluminum * (T_equilibrium - T_aluminum) = m_water * c_water * (T_equilibrium - T_water)
Now we can solve for T_equilibrium:
2.9 kg * 900 J/kg-K * (T_equilibrium - 96°C) = 9.0 kg * 4186 J/kg-K * (T_equilibrium - 5.3°C)
Rearranging the equation and simplifying:
2610 (T_equilibrium - 96) = 37674 (T_equilibrium - 5.3)
2610 T_equilibrium - 250560 = 37674 T_equilibrium - 199250.2
-35064 T_equilibrium = -44810.2
T_equilibrium ≈ 1.28°C
Therefore, the equilibrium temperature of the lump of aluminum and the water is approximately 1.28°C.
Learn more about equilibrium Visit: brainly.com/question/984092
#SPJ11
Can anybody help me with this?

Answers
The correct order of the given elements above in their increasing atomic radii are as follows:
Phosphorus CobaltRutheniumOsmiumGalliumWhat is meant by the atomic radius of an element?The atomic radius of an element is one periodic properties of elements which describes the total distance between the center of the nucleus of an element to the outermost shell of an electron.
From the task given above, the atomic radii values of the elements in the problem above are: Phosphorus ( 98pm ), Cobalt ( 152pm ), Ruthenium ( 178pm ), Osmium ( 185pm ) and finally Gallium which is 187pm.
That being said, below are some few examples of periodicities which is seen in elements in the periodic table:
Melting and boiling pointIonization energyElectron affinityElectronegativityElectrical and thermal conductivityIonic sizeIonic radiusIn conclusion, the atomic radius and atomic size are both periodic properties of elements.
Read more on atomic radii:
https://brainly.com/question/15255548
#SPJ1
A 1.00 g sample of glucose, C6H 1206, is burned in a bomb calorimeter, the temperature of the calorimeter rises by 9.40 0 C. What is the heat capacity of the calorimeter
Answers
According to the question the heat capacity of the calorimeter is 0.449 J/°C
What is calorimeter?A calorimeter is a device used to measure the amount of heat generated or absorbed during a chemical or physical process. It is used in a variety of laboratory experiments to measure the heat of a reaction or the specific heat of a material. The calorimeter is typically composed of a thermally insulated container with a thermometer and a stirrer to mix the reaction and measure the temperature change. The container is usually filled with a known amount of water, and the heat produced or absorbed during the reaction is calculated by measuring the temperature change of the water.
The heat capacity of the calorimeter can be calculated using the equation, q = mcΔT,
where q is the heat energy transferred,
m is the mass of the sample,
c is the heat capacity of the calorimeter, and
ΔT is the change in temperature.
Therefore, the heat capacity of the calorimeter can be calculated as follows:
c = q/(mΔT) = (1.00 g x 4.184 J/g°C) / (9.40°C) = 0.449 J/°C.
To learn more about calorimeter
https://brainly.com/question/2963957
#SPJ4
How many atoms are in 0.96 mol S?
Answers
Answer:
1???
Explanation: